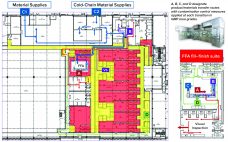
Over the past few years, Oxford Biomedica UK has developed and implemented its fill–finish platform at its 84,000-ft2 “Oxbox” manufacturing facility constructed in 2019. The first phase of development (45,000 ft2) houses four segregated suites for producing bulk viral-vector drug substance (VS) where closed systems and bioburden-control processes apply, and two fill–finish suites for viral-vector drug product (VP) in aseptic processing. The first of the fill and finish suites is expected to be approved in the first half of 2022.…
Manufacturing
eBook: Creating CMC Strategies for Pandemics and Beyond — Perspectives on the Impact of the COVID-19 Pandemic
The COVID-19 novel coronavirus pandemic has highlighted the biopharmaceutical industry’s need to create and implement chemistry, manufacturing, and controls (CMC) strategies that can expedite drug development during times of crisis. This bold topic was the premise of the 2021 CASSS Well-Characterized Biotechnology Products (WCBP) forum titled “Special Edition: Creating CMC Strategies for Pandemics and Beyond.” Held virtually on 25–28 January and 1–4 February 2021, the eight-day event addressed manifold considerations for SARS-CoV-2 virus prevention, diagnosis, and treatment. Five hundred attendees…
eBook: Cell and Gene Therapies — Optimization Strategies for Processing and Potency
Although relatively new to the biopharmaceutical industry, cell and gene therapy development and manufacturing are advancing rapidly. At Informa Connect’s September 2021 Cell & Gene Manufacturing & Commercialization Conference and Exhibition, held in Boston and online, presentations reviewed concerns that arise when processing complex therapies and highlighted some innovative strategies for surmounting those obstacles. Most of those approaches described during the event used data-driven solutions, with each step building on the information gained from the previous one. High-throughput technology platforms…
eBook: Vaccines Revisited — The Past, Present, and Future of Nucleic-Acid Vaccine Production
The quarterly BioProcess Insider eBook series launched in 2021 to investigate specific modalities and business strategies driving the biopharmaceutical sector based upon trends and discussion points presented in the Insider’s pages. Two of the four quarterly publications — this one included — have focused on the vaccine industry. If the series had launched in 2019, cell and gene therapies might have warranted a double edition, or perhaps investments in antibodies and antibody fragments would have driven a bispecific focus. Five…
Optimizing the AAV Transfection Process in Suspension Cells
Given the potentially curative nature of gene and gene-modified cell therapies and the successful launches of several such products over the past five years, markets and investments are growing significantly in the sector. In the United States alone, the US Food and Drug Administration has granted approval for several genetic therapies, including Strimvelis (autologous CD34+, developed by GlaxoSmithKline and later sold to Orchard Therapeutics) in 2016 Luxturna (voretigene neparvovec, Spark Therapeutics), Yescarta (axicabtagene ciloleucel, Kite Pharma/Gilead), and Kymriah (tisagenlecleucel, Novartis)…
Formulation, Fill and Finish of Lentiviral Vectors: Part 1 — Case Study in Facility and Process Design
Over the past few years, Oxford Biomedica Ltd. (OXB) has developed and implemented a fill–finish platform (“Oxbox,” Figure 1) at its viral vector processing facility in the United Kingdom. The facility includes four segregated bulk viral-vector drug substance (VS) suites, where closed systems and bioburden control processes apply, and two viral-vector drug product (VP) fill–finish suites that apply aseptic processing, with space for expansion by scale-out as product output demand increases. Segregated suites enable the facility to process different viral…
Spurring on Innovation in Gene Therapy Development
Gene therapies based on adenoassociated virus (AAV) vectors hold promise for treating myriad conditions. Immunogenicity remains a challenge for such products, however. With support from PerkinElmer, Roland W. Herzog (professor of pediatrics and Riley Children’s Foundation professor of immunology at the Indiana University School of Medicine) joined Nagendra Venkata Chemuturi (scientific director of global research for drug metabolism and pharmacokinetics, DMPK, at Takeda Pharmaceuticals) to deliver a BPI “Ask the Expert” presentation exploring strategies for minimizing immune responses to AAV-based…
eBook: Speed to IND — Practical Wisdom for Uncertain Times
Even in “normal” times, companies need to balance time to filing an investigational new drug (IND) application against careful consideration of processes that can have far-reaching consequences on the quality of biologic products. But supply-chain interruptions still feature prominently in the news during the ongoing COVID-19 pandemic. From equipment to chemicals to plastic components, end users, their suppliers, and (critically) their suppliers’ suppliers all are feeling growing uncertainty about production timelines and availability of materials. In this eBook, BPI’s editor…
Designing Vaccines: The Role of Artificial Intelligence and Digital Health, Part 1
According to the founder and executive chairman of the World Economic Forum, Klaus Schwab, the fourth industrial revolution began in the 21st century and is characterized by an unprecedented development and exponential growth of a high-technology industry transforming society at every level (1–4). In particular, healthcare systems are evolving rapidly to adapt to the new reality. According to Forbes, the main technologies currently shifting the paradigm of medical research are artificial intelligence (AI) and machine learning (ML) (5), both defined…
Transfection Best Practices for AAV Gene Therapy Programs
As viral vectors continue to push gene therapy innovations closer to market, many researchers are setting their sights on optimizing transfection, the process of delivering corrective genetic material into cells. It’s not just a question of how to transfect them, but also how to do so efficiently and at high volumes. Approaches that work for one cell line might not perform well for others, and transfection protocols can have different implications for scalability and cost during production for clinical trials.…