The COVID-19 pandemic has brought myriad economic disruptions, social complications, and public-health calamities to the world. They have understandably overshadowed the silver lining of boosting biomedical science and technology in the realms of infectious disease, oncology, and more. But alongside the much-publicized commercial debut of novel vaccine technologies have come promising advances in medical diagnostics. In this eBook, BPI’s senior technical editor brings together perspectives from industry, academia, and expert organizations to highlight some of the latest diagnostic methods and…
Laboratory Equipment
Tools to Support COVID-19 Patient Testing
To prepare adequate healthcare measures in the case of a pandemic, vast numbers of people must be tested in order to understand the dynamics and behavior of the infection cycle. The medical staff working under extreme circumstances need basic but reliable laboratory supplies to help contain the pandemic. Read this special report to discover two solutions for COVID-19 patient testing that comply with guidelines from the US Centers for Disease Control and Prevention (CDC) and World Health Organization (WHO). The…
Accelerating the Development and Manufacture of Therapeutics Using the Octet Platform
The high costs of therapeutic discovery, development, and manufacture require improved process efficiencies and economics. Analytical tools that eliminate the need for reagent labeling and enable real-time data visualization save development time and improve efficiencies during process development. The Octet biolayer interferometry (BLI) platform and assays can be used throughout process development and manufacturing, including cell-line development, clone selection, and dynamic binding capacity (DBC) determination for affinity purification columns. The ability of the Octet BLI platform to monitor binding interactions…
Measuring Viral Titer in AAV-Mediated Gene Therapy Using a PCR Technique for Absolute Quantitation
Gene therapies have reemerged as promising treatments to a number of genetic illnesses. Nearly 400 gene therapy clinical trials are recruiting or ongoing in the United States for diseases such as hemophilia and spinal muscular atrophy (1). The primary vehicles used today to deliver such therapies to patients’ cells are viral vectors such as adenoassociated viruses (AAVs), but producing biologically active vectors for gene therapy can be problematic. One difficulty is generating vectors at the correct concentration to yield a…
Cell-Line Development: Accelerating Antibody Discovery By Monitoring Titer and Glycosylation with the Octet Platform
Cell line development involves the screening of thousands of clones in an effort to find the few optimal clones that are stable, grow as expected, and produce high yields of the bioproduct. The time it takes from engineering an optimal cell line to the production of the target biologic can be prohibitive and may differ from molecule to molecule. While expression-level analysis like titer screening is carried out early, other critical quality attributes (CQAs) such as glycan characterization are often…
Expanding the Flow Cytometry Toolkit with Next-Generation Polymer Dyes
Since the emergence of SARS-CoV-2, scientists have discovered more about the role of our immune systems and cytokine-associated processes responsible for systemic immune reactions that are typical in patients with COVID-19 (1). Flow cytometry is used to understand those processes at a single-cell level. It is the standard method used in immunology to characterize multiple phenotypic and functional parameters of single cells, including cytokine analysis. Despite advances in flow cytometry instrumentation, reagents, and analytical software, several challenges remain. Conjugated antibodies…
Introduction: Technologies Converge in Biopharmaceutical Laboratories
Bioassay development is foundational to the well-characterized biotechnology product paradigm. Bioassays are the best tools for drug developers to use in determining the biological activity (potency) of their products, which has been a biopharmaceutical critical quality attribute (CQA) since long before that concept had a name. Thus, these assays are vital to quality assurance and quality control (QA/QC), preclinical studies, and clinical testing — and by extension to process development and monitoring. Because of their complex nature, bioassays are among…
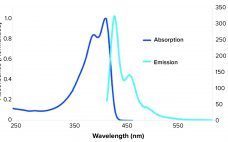
Fluorescent Nanosensors: Real-Time Biochemical Measurement for Cell and Gene Therapies
Cell and gene therapies are destined to transform the methods by which global healthcare challenges are approached and overcome (1). The US Food and Drug Administration is reviewing and approving an increasing number of cell and gene therapy products (2), and biopharmaceutical developers are dedicating immense resources to realizing the enormous potential of these therapeutics. Therefore, technologies that facilitate their effective and efficient manufacture will accelerate cell and gene therapies’ transition from medicines of the future to medicines of the…
Ask the Expert: Best Practices for Aseptic Sampling from Stainless-Steel Equipment
Turn-key single-use aseptic sampling devices (ASDs) have diminished bioprocess contamination risks significantly. But depending on testing, facility, and storage needs, some ASD container types are more effective than others are. Bobbi Allen (technology expert at Sartorius Stedim Biotech North America, SSB) focused her 8 January 2020 “Ask the Expert” presentation on “what, why, when, and where” operators must sample aseptically from stainless-steel tanks. Using data from in-house testing of aseptic sampling containers, Allen offered key considerations for sterility, process monitoring,…
Better Bioprinting Ahead: Breakthroughs and Remaining Challenges
Bioprinted organs soon could revolutionize clinical trials, transplantation, and regenerative medicine. But as Chris Lo reminds us in a new GlobalData report (1), several technical hurdles must be negotiated before biopharmaceutical companies can harness three-dimensional (3D) bioprinting for such purposes. BPI explores persistent printing problems and promising solutions below by analyzing Lo’s report alongside commentary from founding editorial advisory board member Bill Whitford (bioprocess strategic solutions leader at GE Healthcare Life Sciences), Lev Gerlovin (vice president in the life sciences…