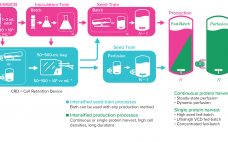
At least one report estimates that by 2025, 35% of biologics will be manufactured using enhanced processes, particularly perfusion-based bioprocessing. Results of two innovations that support this need for enhanced processes are examined below. The Thermo Scientific HyPerforma DynaDrive Single-Use Bioreactor (SUB), the latest advancement in SUB technology, offers better performance than other bioreactors and scalability up to 5,000 L. Gibco High-Intensity Perfusion CHO Medium is formulated to provide exceptional performance and ease of use, with capability of sustaining >1…
Cell Culture Media
Enabling High-Performing
Compounded Media Powder Streamlines Cell Culture Media Preparation Operations
Cell culture medium is critical to cell growth, metabolism, and protein expression. It provides for optimum pH, osmolality, and nutrients in an environment that is essential for cell survival, growth, and expression of proteins and/or metabolites and drug-substance modalities of interest (1). A complete medium typically contains basic nutrients such as carbohydrates, amino acids, lipids, salts, vitamins, trace metals, growth factors/hormones (e.g., insulin), antishear factors, and other chemicals that facilitate cell growth and protein expression and may stabilize recombinant protein…
Reducing Risk in Bioproduction with Facilities Equivalency
Providing consistency in cell culture media biomanufacturing is critical to supply continuity. Central to this is the development of redundancy and harmonization across a global manufacturing network. These unprecedented times have also highlighted the importance of strategizing for increased and unexpected demand. Read this Special Report to learn about the importance of equivalency and the strategies used to maintain this critical requirement at Gibco cell culture media manufacturing facilities. Fill out the form below to read the complete report and…
Using Peptones to Achieve Diverse and Demanding Bioproduction Goals
As bioproduction requirements advance, it is critical to have consistent, high-quality media and supplements that continue to meet evolving industry needs. Peptones have been successfully used in bioproduction applications for more than 30 years to meet diverse and demanding production requirements. Their unique nutritional profiles and usage flexibility make peptones ideal components for creating a robust bioprocess. This Special Report will demonstrate the benefits of peptones and how they can be used to enhance process performance and consistently yield a…
Ask the Expert: Cell Culture Media Analysis Using Handheld Raman Analyzers
In biopharmaceutical manufacturing, cell culture media supply critical nutrients and maintain pH and osmolality to optimize protein product yield. Because media composition and condition have a strong effect on final biologic product quality and production, biopharmaceutical companies monitor media for lot-to-lot variability. Stability testing for degradation due to light exposure, temperature changes, or shelf-life/time is possible with rapid spectroscopic methods. In an 8 October 2019 “Ask the Expert” webinar, O. Dean Stuart (product manager at Thermo Fisher Scientific) explained how…
Upstream: Make the Right Decisions for Your mAb
Bioprocess decisions made during upstream operations can be difficult to reverse at later, more costly stages of biologic manufacture. They even can require significant backtracking, wasting precious time, labor, and material. Read this Special Report to learn ways to optimize monoclonal antibody bioprocessing upstream. Specifically, you will learn about different tools that small and emerging biotechnology groups can use to ensure robust cell-line selection novel media formulations designed for intensified upstream processing in perfusion modes mixing and delivery solutions that…
Making Media a Priority: An Interview with Susan Riley of Advanced Bioprocessing
Susan Riley is vice president and general manager of Advanced Bioprocessing. It’s been a year since Thermo Fisher Scientific’s acquisition of the Advanced Bioprocessing business from Becton Dickinson (BD). Why did Thermo Fisher see the Advanced Bioprocessing (AB) business as a good fit with its life-science offerings? AB has a significant portfolio in premium supplements for cell culture and microbial fermentation. The AB business was seen as a good fit for several reasons: It goes hand-in-glove with Gibco media, for…
Innovative Strategies for Cell Culture Media Preparation
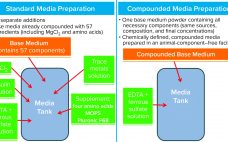
Although the handling and preparation of cell culture media can seem routine, a number of risks are associated with such operations. Identification and mitigation of associated risks can help ensure consistency of performance, minimize likelihood of contamination, and protect employees while enabling greater efficiencies in upstream processes. Here we describe a number of strategies for reducing risks and streamlining media-related workflows. Simplifying Handling of Cell Culture Powders Media preparation typically is quite labor intensive and poses risks related to containment…
The Critical Role of Media in Intensified Upstream Processes
As the need for novel therapeutics increases, so does pressure on the biopharmaceutical industry to improve productivity, accelerate development, increase, and reduce costs — all while ensuring drug product quality. Upstream intensification strategies such as perfusion culture can address those challenges and achieve higher protein titers that can translate into higher throughput, improved flexibility, and compressed timelines. Successful implementation of perfusion culture or the transition to perfusion from fed-batch culture requires a different and strategic approach to media selection, not…
Streamlined Serum-Free Adaptation of CHO-DG44 Cells: Using a Novel Chemically Defined Medium
Monoclonal antibodies (MAbs) have radically transformed the treatment of many chronic diseases, mainly in the fields of oncology and autoimmunity. The overwhelming majority of therapeutic MAbs are manufactured from recombinant Chinese hamster ovary (CHO) cell lines. The original CHO cell line was isolated in the 1950s, and since the early 1980s, it has become the workhorse of the biopharmaceutical industry. The CHO-DG44 strain was generated after several rounds of mutagenesis that deleted both copies of dihydrofolate reductase (dhfr) genes by…