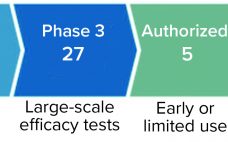
Significant growth of the cell and gene therapy (CGT) pipeline in recent years demonstrates the enormous potential of these modalities to treat or even cure otherwise intractable diseases. Several CGT products have been approved for clinical use over the past five years. More than 75 such products have come to the market around the world so far. They include chimeric antigen receptor (CAR) T-cell therapies that involve genetic engineering of patient cells ex vivo as well as in vivo gene therapies…
Business
Maximizing European Market Access: Guidance for Young Biopharmaceutical Companies
Achieving efficient and profitable market access for next-generation pharmaceutical products is extremely challenging. The number of drug launches is rising every year, taking competition levels higher with them. And because these novel products tend to be more tightly targeted to smaller patient populations than the “blockbuster” drugs of old, their pricing/reimbursement terms need to be tailored to match. This is especially the case with highly complex biologic drugs, which typically are expensive to research and develop. Below I offer a…
Bioprocessing Facilities in Asia Consider Domestic Alternatives to Western Suppliers
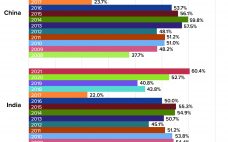
The global biopharmaceutical industry had been growing robustly even before the COVID-19 pandemic. According to the BioPlan Associates Top 1000 Biofacility Index and Biomanufacturers Database (1), bioprocessing capacity worldwide increased an average of 12% over the past decade. China has seen nearly double that rate. India’s bioprocessing segment also is showing strong growth. Because those regions represent 37% of the world’s population — and with rapidly growing middle-class economies, — demand for biologics there is outstripping that elsewhere. Historically, Western…
In Search of a CMO for My Biotechnology Startup: How to Navigate a Journey Without Process Maps
An innovative biopharmaceutical product can transform from an abstract idea at small scale into the basis of a burgeoning startup company. At that point, company leaders seek ways to ensure that a biologic will scale up in a quality-controlled, professional, and sustainable environment. That involves refining a research-stage prototype into a product that will be consistent and reproducible for research and development (R&D) and manufacturing and that will meet all relevant regulatory standards in specified target markets. Within the constraints…
The Biosimilars Action Plan: Promoting Faster and More Extensive Adoption of Biosimilar Drugs
The pace with which biosimilar drugs have been adopted in the United States has frustrated (and displeased) policymakers (1). After passage of the Biologics Price Competition and Innovation Act (BPCIA) (2) as part of the Affordable Care Act of 2010 (3), policymakers intended and expected significant reductions in expenditures for this class of biopharmaceuticals (4). The Federal Trade Commission (FTC) had predicted that the percentage of savings would be lower than that of the <90% reduction in costs for small-molecule…
Collaboration Agreements: Critical Issues and Common Pitfalls
The number of collaboration arrangements for emerging technologies is increasing significantly, especially among companies developing COVID-19 diagnostics, vaccines, and therapeutics. Consequently, a massive amount of capital was invested in the biopharmaceutical industry in 2020. These deals range in value from the low millions to several billion dollars, representing significant risks and value for investors. Some recipients of that capital are seeking partners with whom to collaborate, sharing both the costs and risks associated with their activities and bringing innovative technologies…
Implementation of Established Conditions: Learnings from a “Sharing Science Solutions” Workshop
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline Q12 (1) (step 4 sign-off in November 2019) is in the process of being implemented in a number of regulatory regions. The document provides additional frameworks for pharmaceutical life-cycle management. It is intended to support globally harmonized regulatory tools such as established conditions (ECs) and product life cycle management (PLCM) documents to facilitate postapproval changes to chemistry, manufacturing, and controls (CMC). Although a harmonized framework…
Intellectual Property and COVID-19
The COVID-19 pandemic is the greatest global health threat caused by a virus since the influenza pandemic of 1918 (and, before that, innumerable smallpox outbreaks throughout history). So far, the number of infections and deaths has not reached levels seen during the “Spanish flu” pandemic. However, travel, global trade, and modern factors such as misinformation on social media have increased infection rates and risks of infection. As of 15 July 2021, the World Health Organization (WHO) had reported 188,655,968 confirmed…
eBook: Rare Diseases — Biopharmaceutical Challenges Presented By Relatively Small Patient Populations
By definition, an orphan disease affects a small percentage of the population. However, with some 7,000 such conditions identified so far, they collectively have a significant impact on global health. An estimated 350 million people are affected worldwide by a rare disease — altogether more than the population of the world’s third largest country (the United States). Some well-known biopharmaceutical companies are devoted to developing treatments for rare diseases. However, the vast majority of such diseases have no treatments approved by…
Preparing for a Virtual Audit
Audits are a vital quality-management tool of the biopharmaceutical industry. Whether the objective is to verify supplier or partner qualifications, contribute to corrective and preventative actions (CAPA), or fulfill regulatory compliance requirements, conducting proactive auditing is key to successful operations. Over the past year, virtual audits —also known as remote or distance audits — have enabled biopharmaceutical companies to meet compliance and quality assurance demands despite COVID-19–related travel restrictions and social-distancing protocols. Notwithstanding the challenges of virtual audits, the benefits…