Propelled by year after year of record-setting investments and regulatory approvals, cell and gene therapy (CGT) innovators are on track to revolutionize medicine by providing potential cures for many conditions. Now, CGT manufacturers must plan for a future beyond the production constraints of laboratories. What needs to happen now to prepare for a sustainable, commercially viable scale-up process in years to come? To answer that and other important questions about CGT production, my company, the CRB Group, surveyed more than…
Facility Design/Engineering
Formulation, Fill and Finish of Lentiviral Vectors Part 2: Key Decisions and Risk Management
Over the past few years, Oxford Biomedica UK has developed and implemented its fill–finish platform at its 84,000-ft2 “Oxbox” manufacturing facility constructed in 2019. The first phase of development (45,000 ft2) houses four segregated suites for producing bulk viral-vector drug substance (VS) where closed systems and bioburden-control processes apply, and two fill–finish suites for viral-vector drug product (VP) in aseptic processing. The first of the fill and finish suites is expected to be approved in the first half of 2022.…
Formulation, Fill and Finish of Lentiviral Vectors: Part 1 — Case Study in Facility and Process Design
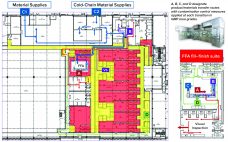
Over the past few years, Oxford Biomedica Ltd. (OXB) has developed and implemented a fill–finish platform (“Oxbox,” Figure 1) at its viral vector processing facility in the United Kingdom. The facility includes four segregated bulk viral-vector drug substance (VS) suites, where closed systems and bioburden control processes apply, and two viral-vector drug product (VP) fill–finish suites that apply aseptic processing, with space for expansion by scale-out as product output demand increases. Segregated suites enable the facility to process different viral…
Aseptic Considerations in Formulation, Fill and Finish: Choosing Between Barrier and Isolator Technologies
Biological drug substances are constituent in a wide range of medicinal products with an even broader spectrum of applications. Those include autoimmune-disease treatments (e.g., for arthritis), vaccines, and recombinant therapeutic proteins (e.g., for cancer treatment). What such products all have in common is that they are manufactured using biotechnology and other cutting-edge technologies. Biologics are not as physically robust as their small-molecule counterparts. Hence, during biomanufacturing processes, these complex molecules present a number of challenges. Some of the typical shared…
Spontaneous Infection: Did You Leave the Back Door Open to Your Cultivation Suite?
Manufacture of biopharmaceuticals using mammalian cells inherently incurs a risk of viral contamination during cell cultivation. If introduced, viruses can infect and replicate in cells used to produce a therapeutic protein or vaccine. The consequences of such contaminations can be dramatic. Not only can a company lose contaminated batches, but it also faces potentially extensive root-cause investigations, facility cleanup efforts, and introduction of preventive measures. Until contamination issues are resolved adequately, production should not be resumed, and facility downtime brings…
Financial Benefits of Off-Site Prefabricated Cleanroom Infrastructures
Traditional cleanroom infrastructures, gypsum, or monolithic wall panels have been used in the past with varying success and benefit. One of the most often proclaimed benefits is the cost of those on-site built structures. Characteristically, the cost quoted at the beginning of a construction project seems to be attractive, but construction estimates are not better than ±50% at feasibility (preconcept) and ±25% at end of conceptual design. Given the above accuracy ranges, industry surveys establish that in most cases, the…
Four Design Factors Shaping Multimodal Cell and Gene Manufacturing
Cell and gene therapy manufacturing is about to hit a breaking point. The tension lies between increasingly diverse research pipelines and a tradition of dedicated facilities built for single-product, large-scale manufacturing. That incompatibility is widening as more cell and gene therapy products progress toward commercial production, forcing manufacturers to make a choice: either invest in major facility modifications and complex technology transfers to keep up or break from tradition and explore the potential of multimodal manufacturing. More than half of…
Toward the Point of Care: Flexibility and Decentralization Are Key to Making Autologous Therapies More Readily Available
Part of the advanced therapy medicinal products (ATMPs) class of therapeutics, cell and gene therapies (CGTs) can be either autologous, using the patient’s own cells, or allogeneic, using master banked donor cells. Global biotechnology company Orgenesis focuses on autologous therapies, with processes and systems developed for closed and automated processing that have been validated for regulatory-compliant production at the point of care for patient treatment. This technology could help overcome the limitations of traditionally cost-prohibitive CGT manufacturing methods that do…
Ask the Expert: Selecting the Right Buffer Management Strategy
Although buffers are among the simplest materials used in bioprocessing, they are critical to biopharmaceutical manufacturing success. Buffer preparation, storage, and handling can require significant investments in time, labor, equipment, and facility space. Jenny Dunker, MSc, and Alexander Troken, PhD (global product managers for customized bioprocess solutions and for process liquids and buffers, respectively, at Cytiva), delivered an “Ask the Expert” presentation on 30 March 2021 to explore strategies for intensifying buffer management. Available Options Biopharmaceutical manufacturers often prepare buffers…
Risk Considerations for Aging Pharmaceutical Facility Cleanrooms
Pharmaceutical facility cleanrooms are designed to reduce and control particle contamination and to minimize the ingress and retention of microorganisms. Such risks typically are easy to control in well-designed, modern facilities. But risk mitigation is more difficult in older facilities. There is no exact definition of what constitutes an aging facility (or what are sometimes euphemistically called legacy facilities). For example, a facility established 100 years ago to manufacture a simple tablet can continue to operate perfectly well with careful…