Adoption of single-use systems (SUS) has increased steadily over the past few years driven by an increased focus on manufacturing biologics that require smaller scales than blockbuster drugs. Although SUS provide many benefits, some factors need to be considered when implementing them in biopharmaceutical production. With the regulatory landscape intensifying worldwide, drug manufacturers are expecting more data from suppliers to support the purity claims of supplied products, especially related to the purity of their product-contact surfaces. In this article, we…
Manufacturing
eBook: Formulation, Fill–Finish — Biopharmaceutical Drug-Product Trends and Technologies
As with many other aspects of biopharmaceutical development, evolving technologies are transforming drug-product development and manufacturing. In a November 2020 report on the Informa Connect “Drug Delivery Partnerships” meeting, BPI senior technical editor Cheryl Scott reviewed how new delivery-device technologies are bringing together companies with disparate expertise to serve patients with chronic conditions better than ever before. The ever-increasing capabilities of electronics and information technology (IT) not only are enabling development of “smart” delivery devices, but also improving the potential…
Reducing Risk in Bioproduction with Facilities Equivalency
Providing consistency in cell culture media biomanufacturing is critical to supply continuity. Central to this is the development of redundancy and harmonization across a global manufacturing network. These unprecedented times have also highlighted the importance of strategizing for increased and unexpected demand. Read this Special Report to learn about the importance of equivalency and the strategies used to maintain this critical requirement at Gibco cell culture media manufacturing facilities. Fill out the form below to read the complete report and…
Optimization of Processes and Advanced Platforms for Viral Vector Processing
Viral vectors are synonymous with gene therapies, so their development, production, and processing are of upmost importance to all gene therapy researchers and manufacturers. Every year, I look forward to attending the Cell and Gene Bioprocessing and Commercialization conference in Boston and talking to leaders in industry and academia about their current approaches to advancing gene therapies. Like most other meetings this year, the conference was entirely online and had to provide a shortened agenda. Nonetheless, there was no shortage…
Manufacture and Regulation of Cell, Gene, and Tissue Therapies, Part 1: Chemistry, Manufacturing, and Control Challenges
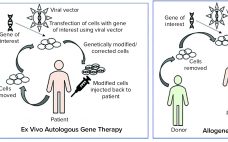
Cell, gene, and tissue (CGT) therapies and other advanced-therapy medicinal products (ATMPs) have made tremendous progress over the past decade. They are different from other biologics and small molecules because of their inherent complexity and variability. Although many unknowns remain about the development of these products, their clinical success has enabled the CGT therapy and ATMP fields to advance rapidly. We are seeing an increase in the number of marketing authorization applications (MAAs) filed in the European Union and new…
Next-Generation Biotechnology Product Development, Manufacturing, and Control Strategies, Part 2: Process Modeling and Analytics
Part 1 of this article focused on the first two sessions of a CASSS chemistry, manufacturing, and controls (CMC) forum entitled “Next-Generation Biotechnology Product Development, Manufacturing, and Control Strategies,” which took place on 16–17 July 2018 in Gaithersburg, MD. Those sessions focused on upstream and downstream process technologies and strategies (1). Part 2 highlights the final two conference sessions on process modeling, control, and analytics. Process Modeling and Control The third conference session was “Modeling and Control Strategies.” Advancements in…
Practical Considerations for Statistical Analyses in Continued Process Verification
Several statistical techniques can be used to assist in monitoring biopharmaceutical product quality attributes as part of continued process verification (CPV) activities. These include run charts, control charts, and capability analyses. Below, I provide an overview and recommendations on statistical strategies when developing a CPV program, considering the expected behavior of manufacturing results in the biopharmaceutical industry. Presence of Autocorrelated Data In a previous study, I highlighted the tendency for data to be positively autocorrelated (values are closely related to…
Evolving Business Models to Meet the Future of Healthcare
Yourway provides integrated supply-chain solutions for the global pharmaceutical and biotechnology industries. We exceed traditional offerings to provide customized support that includes warehousing and packaging, project management, planning and optimization guidance, comparator sourcing, ancillary-supply sourcing, forecasting, and returns/reconciliation management. Our single–specialized-provider approach offers clients a high level of convenience and efficiency that translates into quality, speed, and operational improvements. A Growing Global Footprint As we introduce new added-value solutions, expand our global footprint, and continue investing in infrastructure, technology, training,…
Case Study for a Facility-Fit Driven Process Development
Time to clinic and time to market are the key drivers for client success in the biopharmaceutical industry. Facility fit is becoming key to understanding process constraints and which aspects of the process have the largest impact on enabling facility fit. Process development with facility-fit constraints in mind will ensure a smooth technology transfer and shorten the timeline of current good manufacturing practice (CGMP) product delivery to clients. Fill out the form below to read this Special Report and learn…
Cryopreserved Leukapheresis Materials Help Alleviate Donor Sourcing Issues
Reliable access to high-quality starting material is a primary challenge in cell therapy manufacturing. Freshly isolated leukopak starting materials depend on donor availability and are vulnerable to cell losses from scheduling changes and other unforeseen circumstances. Flexibility often is required for cell therapy manufacturing. Therefore, relying solely on freshly isolated starting material is impractical, particularly when cell therapy logistics involve global shipping and distribution. Donor sourcing is among the most critical factors shaping cell and gene therapy (CGT) supply chains.…