Single-use fermentors (SUFs) offer dramatic advantages over traditional stainless-steel clean-in-place (CIP)/sterilization-in-place (SIP) fermentors. Single-use technology eliminates the need for steam, chemicals, and water to clean and sterilize stainless vessels, which provides a direct reduction in capital cost and environmental-impact mitigation. Single-use platforms also increase equipment and process flexibility significantly, making it possible to switch campaigns from one product to another in minimal time, while eliminating cleaning and associated validation steps (1). Both Cytiva and Thermo Fisher Scientific offer SUFs that…
Upstream Processing
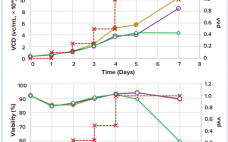
High-Yield Production of rAd26-S for Sputnik V Vaccine Component I: An Optimized Process in a Scalable Shaken Bioreactor
The recent outbreak of the severe acute respiratory syndrome coronavirus (SARS-CoV-2) led to the development of different vaccine approaches worldwide to prevent the coronavirus disease 2019. The first registered vaccine on the market was the Sputnik V product based on two recombinant adenoviral vectors (Ad5 and Ad26). The product has received approval in 70 countries by several national and regional regulatory authorities, meanwhile. Though the availability of SARS-CoV-2 vaccines in most developed countries is not an issue any longer, other…
Gain Control of Culture Conditions: Technology for Sustained Delivery of Recombinant Proteins
Growth factors, added at the precise time and concentration to in vitro cultures are essential to control cell proliferation and differentiation. When added to a culture vessel, however, the concentrations of these signaling proteins rapidly decline, altering both levels of individual growth factors and the ratio of factors for cell signaling. When growth factors are replenished by exchanging the medium, concentrations peak, resulting in an ebb and flow of growth factor levels resulting in mixed signaling that can lead the…
A Strategic Approach to Selecting the Optimal Process Intensification Scenario
Current demands placed on the biopharmaceutical industry are pushing manufacturers toward process intensification, an approach that modifies unit operations or an entire manufacturing process to optimize efficiency. Three common intensification scenarios in upstream processing are seed-train intensification (usually at the n – 1 stage), concentrated fed-batch production, and dynamic perfusion (at the production bioreactor stage). In downstream processes, intensification strategies typically involve moving from single- to multicolumn chromatography. Biomanufacturers can realize several kinds of improvements from intensified processing, including reductions…
Spontaneous Infection: Did You Leave the Back Door Open to Your Cultivation Suite?
Manufacture of biopharmaceuticals using mammalian cells inherently incurs a risk of viral contamination during cell cultivation. If introduced, viruses can infect and replicate in cells used to produce a therapeutic protein or vaccine. The consequences of such contaminations can be dramatic. Not only can a company lose contaminated batches, but it also faces potentially extensive root-cause investigations, facility cleanup efforts, and introduction of preventive measures. Until contamination issues are resolved adequately, production should not be resumed, and facility downtime brings…
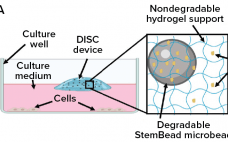
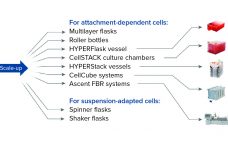
Strategizing Scale-Up and Scale-Out for Cell Therapy Production
When considering strategies for expanding the number of cells being grown to support cell therapy development, companies often focus on decisions regarding scale-up and scale-out: increasing capacity either by using larger vessels to increase production volume or by implementing more units of the same vessel, respectively. Complete workflows often involve both. Figure 1 shows an example of scaling out from one to multiple cell culture flasks of the same dimension before transitioning to a larger format. Scale-out can be straightforward…
Ask the Expert: Facilitating Process Development Using a Microfluidic Perfusion Bioreactor
Suitable scale-down perfusion systems generally have been unavailable for process development (PD) activities. Some commercially available systems require daily media exchanges. No such system performs in a way that accurately represents large-scale perfusion, and none maintains sufficiently high cell densities. Kevin Lee (cofounder of Erbi Biosystems) joined BPI on 4 May 2021 to explain how his company’s Breez bioreactor system integrates all the functions of a stirred-tank reactor (STR) into a compact format that can facilitate PD, enabling one person…
Measuring Cell Density in HyPerforma S.U.B.s with ABER Futura neotf
Single-Use Sensors
Monitoring critical process parameters (CPPs) and key performance indicators in bioreactor control systems is crucial to ensure proper cell growth and protein production. Today, most of the major biopharmaceutical companies employ capacitance measurement, in R&D and through process development to manufacturing. Owing to the increased use of single-use bioreactors and building on Aber’s experience with single-use capacitance sensors, the latest Futura neotf single-use capacitance sensors have been specifically developed for integration into Thermo Fisher Scientific bioprocess containers (BPCs) for use…
Avenues for Innovation: The Latest in Cell-Line Engineering and Development
Plato wrote in ancient Greece that “our need will be the real creator,” which transformed over time into the English proverb, “Necessity is the mother of invention.” Advancements in medicine and biomanufacturing technology in 2020 have epitomized that idea. Even as technologies such as mRNA vaccines have rocketed into the public’s awareness, biomanufacturing experts have worked behind the scenes with renewed vigor spurred on by hard lessons from the pandemic. Cell-line development and engineering are no exception. Already undergoing a…
Increasing Expression Titers: New Technologies Could Help Other Cell Lines Catch Up to CHO
Fang Tian is a lead scientist and head of cell biology research and development at the American Type Culture Collection (ATCC) in Manassas, VA. She is a member of both the International Cell Line Authentication Committee (ICLAC) and the US technical advisory group for the ISO/TC276 technical committee. At ATCC, she oversees preparation, authentication, characterization, quality control, and cryopreservation of more than 3,400 accessioned animal cell lines and hybridomas in the cell biology general collection. She holds a PhD in…