The COVID-19 pandemic has shone a light on restrictive business processes, information silos, and poor supply-chain visibility in many sectors. In biopharmaceutical manufacturing, for example, difficulties associated with product-quality management have been exposed and starkly felt. However, public healthcare measures over the past 18 months have put physical distance between team members, thereby hampering the usual form-filling, manual sign-offs and spreadsheet-based recordkeeping associated with monitoring traditional manufacturing processes. In some cases, a lack of formal face-to-face discussions in the workplace…
PAT
eBook: Sensors — Process Analytics for Modern Biopharmaceutical Workflows
To achieve quality by design in biopharmaceutical production, manufacturers need tools that can ensure the stability of critical process parameters (CPPs) and other performance indicators related to product critical quality attributes (CQAs). Over the past couple of decades, sophisticated process analytical technologies (PATs) have emerged to address such needs. Offerings are now abounding for single-purpose sensors that measure temperature, pressure, pH, glucose, protein concentration, or dissolved oxygen. New in-line formats are enabling such instruments to provide data in real time,…
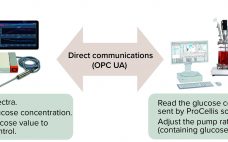
Automated Process Control Based on In Situ Measured Glucose Concentration
A process analytical technology (PAT) strategy involves defining critical process parameters (CPPs) of a biomanufacturing process that influence critical quality attributes (CQAs) and controlling those CPPs within defined limits. Doing that enables consistent product quality and helps companies reduce waste and costs. Glucose is an important CPP in bioprocessing and cell therapy. Glucose often is fed as a bolus addition based on daily off-line measurements, but that can lead to high glucose fluctuations and to excessive glucose feeding, which can…
Seamless Integration of Glucose Control: Using Raman Spectroscopy in CHO Cell Culture
The process analytical technology (PAT) and quality by design (QbD) guidelines promoted by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) support the idea that quality cannot be “tested into” a biologic product but must instead be part of its process design. Seamless integration of analytical data with bioprocess monitoring and control is crucial to understanding a process and overcoming manufacturing challenges that arise in the course of development. Monitoring of product quality attributes (PQAs)…
Simplifying the Bioprocessing 4.0 Journey
Bioprocessing 4.0, the biopharmaceutical version of Industry 4.0, is on course to become a reality in the next decade (1). This is because its “cyber-physical systems” that comprise cloud computing, connected systems, and digital process control offer many benefits. They include better process monitoring and management of a biologic’s critical quality attributes (CQAs) and the chance to control intensified processes for faster, less-expensive production of protein-based biologics and vaccines. Automation also can reduce the number of skilled operators needed while…
PendoTECH Sensors and Industry 4.0: Integrating a PendoTECH Single-Use Sensor System with a Digital Highway
Biopharmaceutical manufacturing is evolving with the progression of Industry 4.0. Industry 4.0 refers to the ongoing “fourth industrial revolution,” which is transforming modern manufacturing and production practices through the use of “smart” technology. This is appealing especially to the biopharmaceutical industry, where production can be a long, meticulous, complex process, and optimizing manufacturing procedures is critical for success. As a leading supplier of single-use technology for the biopharmaceutical industry, PendoTECH recently has explored how its products can be integrated easily…
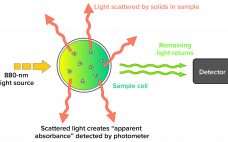
Validation of a Next-Generation Single-Use Turbidity System
Turbidity describes the relative clarity of a liquid as the result of suspended solids. Instruments that measure turbidity typically use a beam of light to detect particles by measuring the difference between the amount of light emitted from the light source and the amount that is received by a detector. Such measurements are affected by the size, shape, and number of particles in a sample of liquid because those solids scatter the incoming light, which provides an apparent absorbance that…
Measuring Cell Density in HyPerforma S.U.B.s with ABER Futura neotf
Single-Use Sensors
Monitoring critical process parameters (CPPs) and key performance indicators in bioreactor control systems is crucial to ensure proper cell growth and protein production. Today, most of the major biopharmaceutical companies employ capacitance measurement, in R&D and through process development to manufacturing. Owing to the increased use of single-use bioreactors and building on Aber’s experience with single-use capacitance sensors, the latest Futura neotf single-use capacitance sensors have been specifically developed for integration into Thermo Fisher Scientific bioprocess containers (BPCs) for use…
eBook: Bioreactor Sensors —
Inside the Dynamics of Cell Culture
Cell culture monitoring can fall into something like a “black box” conundrum. Efforts to measure key parameters such as pH, glucose, and even cell density require sampling and removal of the contents from a bioreactor. But that procedure can expose both a process and an operator to contamination risks. Emerging bioreactor sensors are designed to address some of those challenges, but the rapid adoption of single-use technologies and the rise of perfusion cell culture have presented obstacles to their implementation.…
How Capacitance Measurement Can Improve Viral Vector and Virus-Based Vaccine Production
With the increasing development of viral vector and virus-based vaccines, technologies that help to manufacture and scale up these types of vaccine quickly and cost-effectively have become more critical. By accessing this special report from Aditya Bhat, a capacitance technology expert at Aber Instruments, you will find out how in-line capacitance measurement can produce a detailed fingerprint of cell culture processes and how that can benefit vaccine production. From case studies involving baculovirus, AAV, and measles, you will discover why…