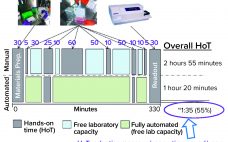
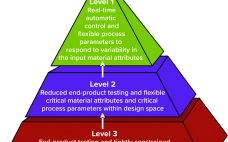
The commercial manufacturing success of monoclonal antibodies (MAbs) has become a touchstone of the biopharmaceutical industry. MAbs are so well established that they often are referred to as “traditional” biologics, and well-known MAb processing methods have become a model for processing of other “advanced” or “emerging” therapies. But MAb processing continues to advance as biomanufacturers seek ways to improve efficiencies, lower costs, and (most recently) increase sustainability of facilities. Drug makers are particularly interested in strategies for MAb process intensification.…
Author Archives: Maribel Rios
eBook: Cell and Gene Therapies — Optimization Strategies for Processing and Potency
Although relatively new to the biopharmaceutical industry, cell and gene therapy development and manufacturing are advancing rapidly. At Informa Connect’s September 2021 Cell & Gene Manufacturing & Commercialization Conference and Exhibition, held in Boston and online, presentations reviewed concerns that arise when processing complex therapies and highlighted some innovative strategies for surmounting those obstacles. Most of those approaches described during the event used data-driven solutions, with each step building on the information gained from the previous one. High-throughput technology platforms…
Preparing for Process Improvements: Discussions of the Cell Therapy Industry’s Supply Chain, Automation, and Control Needs
As editors, we are fortunate to have the opportunity to listen to biopharmaceutical developers and innovators discuss the intricacies of their work. Typically, we find that the best discussions come from asking two fundamental questions: What need did you observe in the industry that drove your work, and what technologies would help you do your job better? Over the past five years or so, the answers have shifted. Cell and gene therapy (CGT) innovators are focusing on increasingly complex diseases…
Automation of Potency Assays: A Strategic Journey
Cell-based potency testing provides quantitative data concerning a drug’s biological activity. Thus, it plays an essential role in biopharmaceutical quality control (QC), good manufacturing practice (GMP) product release, comparability determination, and stability testing for both drug products and drug substances. Potency is a critical quality attribute (CQA) often scrutinized by regulators and reviewers. Test methods are specific to a drug’s mechanism of action (MoA) and should be validated to internationally harmonized regulatory standards (1). The options preclude applying a simple…
Optimization of Processes and Advanced Platforms for Viral Vector Processing
Viral vectors are synonymous with gene therapies, so their development, production, and processing are of upmost importance to all gene therapy researchers and manufacturers. Every year, I look forward to attending the Cell and Gene Bioprocessing and Commercialization conference in Boston and talking to leaders in industry and academia about their current approaches to advancing gene therapies. Like most other meetings this year, the conference was entirely online and had to provide a shortened agenda. Nonetheless, there was no shortage…
November–December 2020:
From the Editors
As 2020 draws to a close, we can’t help looking back at this year in which the biopharmaceutical industry has received more attention than ever before — scientifically, politically, and otherwise. We editors always have been conscious of (and pretty good at) separating our work and home lives. As many of you have learned this year, that’s an important part of working from home, which we’ve been doing for over a decade. But in recent months, reading the news and…
eBook: Viral Vector Purification — A Discussion of Current Challenges and Methods
Adenoassociated viral (AAV) vectors have become synonymous with gene therapy delivery. However, because they are produced in such small quantities and because their upstream processes carry comparatively large amounts of host-cell DNA and other impurities, AAV purification can be challenging. Several researchers have applied different chromatographic strategies, but no universal method has been adopted in the biopharmaceutical industry. This eBook features a discussion among several industry experts that explores challenges specific to AAV purification, shedding light on whether current strategies…
Optimizing Cell Line Development for High-Quality Biologics
For a host-cell system to generate high yields of recombinant proteins and other entities, cells must be derived from optimized and stable cell lines. However, cell line development (CLD) can be tedious and time-consuming work, and every stage in the CLD workflow has its limitations and challenges. Researchers are creating advanced strategies and tools to overcome those challenges, especially for complex biologics such as bispecific antibodies (BsAbs) and difficult-to-express (DTE) proteins. Online presentations from the CLD track of the BioProcess…
Plant-Cell Cultures and Cell Lines for Recombinant Protein Expression
Cell cultures derived from mammalian and bacterial cell lines are the conventional production systems in bioprocessing. But they also have their limitations. Media for mammalian cultures in particular are notoriously expensive, and traditional cell cultures can be highly sensitive to growing conditions. During the late 1980s and into the 1990s, plants and plant-derived cell cultures were introduced as alternative cell-culture systems (1, 2). Although transgenic plants (genetically modified) once looked promising in the early 2000s, the cost and manufacturing complexity…
Developing Process Control Strategies for Continuous Bioprocesses
Process control enables biomanufacturers to ensure that operating parameters are within defined specifications. A control strategy should be established during early stages of process development while process and product performance are being defined using risk-based methods such as quality by design (QbD) and process analytical technologies (PATs). Confirming process control as an essential part of product development creates greater process knowledge and understanding and provides the first steps toward process optimization. By understanding how process performance relates to product quality,…