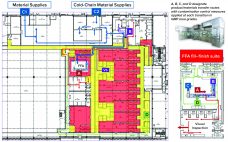
Over the past few years, Oxford Biomedica UK has developed and implemented its fill–finish platform at its 84,000-ft2 “Oxbox” manufacturing facility constructed in 2019. The first phase of development (45,000 ft2) houses four segregated suites for producing bulk viral-vector drug substance (VS) where closed systems and bioburden-control processes apply, and two fill–finish suites for viral-vector drug product (VP) in aseptic processing. The first of the fill and finish suites is expected to be approved in the first half of 2022.…
Author Archives: James L. Drinkwater
Formulation, Fill and Finish of Lentiviral Vectors: Part 1 — Case Study in Facility and Process Design
Over the past few years, Oxford Biomedica Ltd. (OXB) has developed and implemented a fill–finish platform (“Oxbox,” Figure 1) at its viral vector processing facility in the United Kingdom. The facility includes four segregated bulk viral-vector drug substance (VS) suites, where closed systems and bioburden control processes apply, and two viral-vector drug product (VP) fill–finish suites that apply aseptic processing, with space for expansion by scale-out as product output demand increases. Segregated suites enable the facility to process different viral…