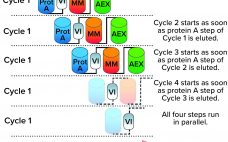
The biopharmaceutical industry generally acknowledges that upstream and downstream aspects of drug-substance manufacturing are experiencing a capacity mismatch. Today, many recombinant proteins can be produced at expression titers of 3 g/L, with some yields exceeding 10 g/L. Such titers represent 100-fold increases in production capability compared with values from twenty years ago (1, 2). Increases in cell-culture density and improvements to perfusion-mode bioreactor systems hold promise for increasing yields further still. Such developments, combined with the broad availability of concentrated…
Separation/Purification
AAV Downstream Process and Product Characterization: Integrating Advanced Purification and Analytical Tools into the Workflow
The optimization of the downstream process for Adeno-associated virus (AAV) production with consistent quality depends on the ability to characterize critical quality attributes affecting potency, purity and safety of the final product. As the gene therapy field continues to push products through the clinical pipeline, an increasing need for efficient purification and analytical tools has become evident. In addition, the regulatory space has expanded in parallel to the use of AAV, driving the demand for simple and efficient assays to…
Chromatography in mRNA Production Workflow
Rapid response to global pandemics requires the manufacture of billions of vaccine doses within months. This short timeline must allow for design and testing of active ingredients, development of production and purification processes, clinical evaluations, regulatory filings, and manufacturing. Existing purification methods often have been adopted from laboratory-scale techniques to allow rapid implementation, and those have provided adequate product quality. But future mRNA development will require optimized production and purification processes. Chromatography has been a workhorse of biomanufacturing for decades,…
Purity By Design
Astrea Bioseparations has a well-established modular program to support customer projects from small to large scales with ligands, adsorbents, and chromatography columns that design purity into each process. Demand for increased productivity in biopharmaceutical manufacturing has placed new pressure on downstream purification operations. For recombinant proteins and monoclonal antibodies (MAbs), such pressure stems from significant gains in upstream productivity, particularly from high titers produced using increasingly efficient cell-culture systems. For viral vectors used in gene and gene-modified cell therapies and…
Reducing Cell and Gene Therapy Development Time and Cost with New Purification Strategies
The past 40 years have ushered in the most advanced medicines the world has ever seen, with tremendous improvements in biomanufacturing technologies to enable their development. Advances in production technology have brought significant improvements in upstream productivity, which then caused bottlenecks in downstream processing. Although many bottlenecks have been resolved for most biologics, new modalities such as gene therapies and mRNA vaccines are driving the need for differentiated purification solutions. Meanwhile, pressures to increase efficiency and reduce costs continue to…
Risk Determination of Potential Mycotoxin Exposure to Patients: Testing Recombinant Human Factor VII from Transgenic Rabbits
Sevenfact eptacog beta is a new recombinant human factor VIIa (rFVIIa) developed by LFB SA in Les Ulis, France, as a bypassing agent (BPA) for treatment and control of bleeding in people with hemophilia A and B and inhibitors (1, 2). The product was approved for use in adults and adolescents by the US Food and Drug Administration (FDA) in April 2020 (3). It is expressed in the milk of transgenic rabbits and purified through a multistep process using both…
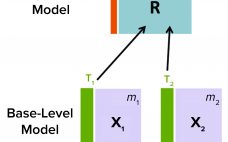
Advanced Data-Driven Modeling for Biopharmaceutical Purification Processes
Purification is an essential process in biopharmaceutical manufacturing that separates a therapeutic protein in its active form from impurities. A typical purification process consists of several chromatography unit operations, and each unit operation comprises multiple phases. During the operation of each step, continuous (time-series data per parameter for each batch) and batch data (one data point per parameter for each batch) are generated by in-line sensors installed in chromatography skids on the production floor and with at-line/off-line in-process samples, respectively.…
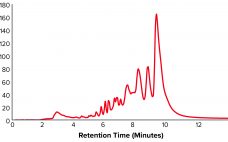
Development of a Universal Preparative Anion-Exchange Method to Purify Oligonucleotides
Oligonucleotide-based therapeutics have been studied over recent decades, and their promise as a new drug modality is now being realized. The growing interest in oligonucleotides is driven by their high potential for treating different medical conditions, the growing number of oligonucleotide drugs approved by the US Food and Drug Administration (FDA), an increased focus on personalized medicines, the development of therapies for rare diseases, and the wide adoption of nucleotide-based COVID-19 vaccines. Oligonucleotides are short, linear sequences of DNA or…
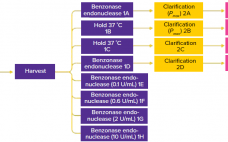
The Effect of Benzonase Endonuclease Addition to Purification of Sabin Poliovirus Type 3
During production of vaccines and viral vectors, the size and quantity of extracellular nucleic acids must be reduced using endonuclease enzymes. Merck/MilliporeSigma’s proprietary Benzonase endonuclease is a genetically engineered nuclease derived from the Gram-negative bacteria Serratia marcescens. It attacks and degrades all forms of DNA and RNA. It is manufactured under good manufacturing practice (GMP) conditions and has a drug master file (MDF) in place with the US Food and Drug Administration (FDA), which can be cited in regulatory filings.…
New Antibody Formats on the Block:
More Complex Modalities Demand Innovation in Manufacturing and Purification
Some of the latest, most promising therapeutic developments in the biologics industry use antibody fragments — either separate functional subunits of antibodies or recombinant molecules that are composed of immunoglobulin domains. The most popular fragments are antigen-binding fragments (Fabs), variable single-chain fragments (scFvs), diabodies, and nanobodies. Such molecules raise several advantages over their parent molecules for upstream production but pose several challenges for downstream purification. To facilitate antibody-fragment capture, Tosoh Bioscience has developed Toyopearl AF-rProtein L-650F resin. Its ligand uses…