The major fluid products used in bioprocessing — culture media and buffers — are classically prepared in-house by rehydrating (dissolving and mixing) powders purchased from suppliers. Most bioprocessing facilities consider in-house preparation of these fluids to be a core bioprocessing task. However, some companies are outsourcing the work either by purchasing preprepared materials from vendors or hiring contract manufacturing organizations (CMOs) to prepare them. Buffer fluid preparation is one area of downstream production operations that are seeing an increase in…
Downstream Contract Services
Emerging Technology Trends in Biologics Development: A Contract Development and Manufacturing Perspective
For a contract development and manufacturing organization (CDMO), process development and manufacturing of recombinant proteins must be linked because of tight timelines driven by client expectations. Those are in turn driven by a need for rapid progression to clinical testing. Early in process development, the choice of raw materials needs to reflect existing supply chain and manufacturing infrastructure, but remain suitable for scaling up to meet future needs. One approach is to establish platform processes for a class of molecules…
A CMO Perspective on Quality Challenges for Biopharmaceuticals
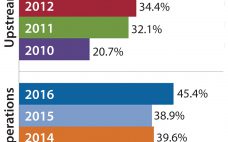
The global annual revenue for biopharmaceuticals has been growing consistently since 2001, accounting for 15.6% of the total pharmaceutical market in 2011. The global biopharmaceutical market was valued at US$138 billion in 2011 and is expected to surpass $320 billion by 2020 (1). The market for recombinant proteins now exceeds $100 billion, a milestone attained in 2011. Figure 1: () Much of the growth in biopharmaceutical revenue is due to an increasing number and sales of recombinant…
Artifacts of Virus Filter Validation
Virus filters are used in biomanufacturing to ensure the safety of biopharmaceutical drug products. As part of filter implementation, manufacturers are required to validate that the filtration process can indeed remove virus. Validations are typically performed at contract testing organizations (CTOs) that are “equipped for virological work and performed by staff with virological expertise in conjunction with production personnel involved in designing and preparing a scaled-down version of the purification process” (1). Virus removal capability of a filtration process is…
Supporting Continuous Processing with Advanced Single-Use Technologies
It has been 10 years since the US Food and Drug Administration (FDA) articulated — in its guidance for process analytical technology (PAT) — the goal of “facilitating continuous processing to improve efficiency and manage variability” (1). Since that time, regulators and industry have worked toward applying continuous processing (CP) to all facets of pharmaceutical manufacturing, including bioproduction (2, 3). Last year, the European Medicines Agency (EMA) referred to CP in its draft Guideline on Process Validation, and the FDA…
Single-Use Technologies in Cell Therapy
Single-use technologies (SUTs) are tools that can be used in producing cell therapies and personalized medicines. Such products must meet specific requirements because of the way they are used. To meet those criteria, the cell therapy industry simply has no alternatives to single-use systems. SUT applications are rapidly changing. Traditional uses for single-use systems in cell therapy include processing in clinical settings (e.g., blood bags, transfer sets) and research and development (e.g., T-flasks, pipettes). Although such applications continue, the commercialization…
Downstream Technology Landscape for Large-Scale Therapeutic Cell Processing
The cell therapy industry (CTI) is poised to grow rapidly over the next decade, treating millions of patients and generating annual revenues into the tens of billions of US dollars (1, 2). To meet that high-growth demand, large CTI system manufacturers (e.g., Corning, Nunc/Nalgene, and GE Healthcare) and leading contract manufacturing organizations (CMOs, such as Lonza) are developing and integrating new upstream technology platforms such as gas-permeable membranes and microcarrier-based bioreactors to significantly increase therapeutic cell culture productivity. As those…
A Statistical Approach to Expanding Production Capacity
Contract manufacturer DSM Biologics — at its current good manufacturing practices (CGMP) facility in Groningen, The Netherlands — provides services for clinical development and commercial production based on mammalian cell culture technology (Photo 1). During the 2011–2012 year, the facility went through a major expansion project to enlarge its capacity and fulfill a growing customer demand. From a business point of view, the project had a well-defined target for future production capacity as well as investment volume. Photo 1: Photo…
Preformulation Development of a Recombinant Targeted Secretion Inhibitor
Our company carried out a preformulation study on a recombinant targeted secretion inhibitor (TSI) with contract research organization (CRO) Avacta Analytical. In this protein, the binding domain of botulinum toxin is replaced to broaden the toxin’s therapeutic potential and allow drug development to be targeted towards a specific disease. In our study, we took advantage of the high-throughput, microvolume protein analysis of Avacta’s Optim 1000 fluorescence and light-scattering instrument (which is distributed in the United States by Pall Corporation). It…
Evaluating Library Databases for Microbial Identification: Critical Aspects and Recommendations
A thorough evaluation process for microbial identification systems should consist of both a technical and financial review, regardless if you are performing internal testing and outsourcing. Assessment of the library database used for microbial identifications is a critical component of evaluating a system or service in its ability to generate accurate identifications. Comprehensive depth of entries, accuracy and coverage of relevant species frequently found in aseptic and sterile manufacturing environments have a significant impact on both performance and cost. Databases…