Recombinant proteins such as growth factors and cytokines are essential for cell therapy, gene therapy and regenerative medicine research, development and manufacturing. These proteins are critical in the production of desired cell types and subsequent differentiation of cells, to deliver the desired effect. Founded 15 years ago, Shenandoah Biotechnology applies a proprietary method of folding and purifying recombinant proteins from both bacterial and mammalian systems to enable cost-effective, large-scale production of Cell Therapy Grade proteins to support these groundbreaking treatments.…
Manufacturing
Aseptic Considerations in Formulation, Fill and Finish: Choosing Between Barrier and Isolator Technologies
Biological drug substances are constituent in a wide range of medicinal products with an even broader spectrum of applications. Those include autoimmune-disease treatments (e.g., for arthritis), vaccines, and recombinant therapeutic proteins (e.g., for cancer treatment). What such products all have in common is that they are manufactured using biotechnology and other cutting-edge technologies. Biologics are not as physically robust as their small-molecule counterparts. Hence, during biomanufacturing processes, these complex molecules present a number of challenges. Some of the typical shared…
Creative Formulation: A Useful Approach to Patient-Centered Drug Development
The development of new medicines is a highly regulated process focused on demonstrating efficacy and safety of new products. Although such qualities always will remain the primary focus of drug development, the biopharmaceutical industry gradually has adopted additional design aspects. New approaches can help meet patients’ divergent needs to improve their lives in meaningful ways. Often referred to as “patient-centered” or “patient-focused” drug-product design, such considerations are expected by many experts to become an increasingly dominant part of future drug…
A Holistic Approach: Bridging the Gap Between Suppliers and Biomanufacturers
How can biopharmaceutical manufacturers expect their suppliers to deliver what the industry wants and needs if it doesn’t communicate those desires? Without industry input, biotechnology suppliers are developing technologies “blindly.” Despite their having delivered great value over the years, together the greater community can do better. Aligned industry input is a vital element in the development process. Here we describe how two BioPhorum workstreams focused on drug-product development have worked to facilitate these communications. Last summer, the container–closure integrity (CCI)…
Closed-System Transfer Devices: Collaboration Provides Tools to Guide Compatibility and Stability Testing Strategy
Ever since the first biopharmaceutical product (biologic) was approved in the 1980s, companies have developed protocols and tests to ensure that such products are safe and effective. Biologics are very different from traditional small-molecule drugs, with unique risks inherent to their manufacturing processes. Biopharmaceutical formulations often present as complex mixtures that can be sensitive to heat, light, and many other factors, all of which must be monitored and assessed. However, until recently, developers worked mostly independently, with only their own…
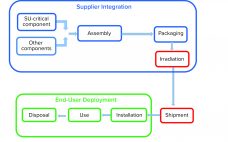
Integrity of Single-Use Systems: Practical Applications and Deployment
Single-use (SU) technology plays an important role in modern vaccine and biologics manufacturing. System integrity, managed by critical process controls, ensures sterility and is a prerequisite for successful leak-free processing. Nonintegral systems cause loss of product, quality, and time; increase costs through investigations; and lead to potential safety problems. The BioProcess Systems Alliance (BPSA) issued a white paper in 2017, Design, Control, and Monitoring of Single-Use Systems for Integrity Assurance (1), that describes in detail the strategies for design and…
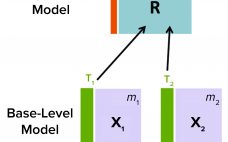
Advanced Data-Driven Modeling for Biopharmaceutical Purification Processes
Purification is an essential process in biopharmaceutical manufacturing that separates a therapeutic protein in its active form from impurities. A typical purification process consists of several chromatography unit operations, and each unit operation comprises multiple phases. During the operation of each step, continuous (time-series data per parameter for each batch) and batch data (one data point per parameter for each batch) are generated by in-line sensors installed in chromatography skids on the production floor and with at-line/off-line in-process samples, respectively.…
Simplifying the Bioprocessing 4.0 Journey
Bioprocessing 4.0, the biopharmaceutical version of Industry 4.0, is on course to become a reality in the next decade (1). This is because its “cyber-physical systems” that comprise cloud computing, connected systems, and digital process control offer many benefits. They include better process monitoring and management of a biologic’s critical quality attributes (CQAs) and the chance to control intensified processes for faster, less-expensive production of protein-based biologics and vaccines. Automation also can reduce the number of skilled operators needed while…
eBook: CAR-T Cell Therapy — Mitigating Clinical and Bioprocess Limitations
Developers of chimeric antigen receptor (CAR) T-cell therapies are working in a state of tempered optimism. As of September 2021, the US Food and Drug Administration has approved only five such products, two coming this year. Now that those approved products have demonstrated the viability of CAR-based immunotherapies, drug developers are trying to address significant limitations that have come to light with increases in available clinical data and bioprocess knowledge. One shortcoming concerns therapeutic efficacy. Blood cancer patients who have…
Single-Use Technology for Formulation and Filling: A Case Study from Swissfillon AG and Pall Corporation
Swissfillon AG is a contract manufacturing organization (CMO) based in Switzerland. Fully compliant with current good manufacturing practice (CGMP) regulations, it provides state-of-the-art aseptic filling for pharmaceutical and biotechnology companies, from clinical-phase materials to commercial quantities. This CMO specializes in high-value, difficult-to-fill products. Swissfillon recognized that adoption of single-use systems (SUS) on a commercial scale required major improvements in consistency and reliability compared to manual operations at pilot and clinical-trial scale. The single-use formulation and filling process, which includes an…