The Chinese hamster ovary (CHO) cell line was first established by Theodore Puck in the 1950s and was used mainly for cytogenetics studies (1). Since the 1990s, CHO cells have increased in popularity as expression host cells because they can be adapted easily into suspension culture and genetically modified. The CHO cell line also has a human-like glycosylation profile (2–4). Therapeutic proteins undergo different posttranslational modifications (PTMs) during manufacturing. Some modifications can lead to undesired effects such as decreased stability,…
Expression Platforms
Ask the Expert: A Novel Escherichia coli System for Controlled Release of Difficult-to-Secrete Proteins
Although Escherichia coli often enables dependable, highly productive expression of nonglycosylated recombinant proteins, the efficiency with which it secretes a target protein into culture supernatant can depend greatly on that molecule’s physicochemical properties. Some proteins remain trapped in periplasm, thus diminishing process yield and productivity. In June 2021, Marcel Thoen (head of Wacker Biotech’s Global Competence Center for Cell Line Development) described how his company’s improved ESETEC (E. coli secretion technology) solutions can address productivity challenges raised by difficult-to-secrete recombinant…
Avenues for Innovation: The Latest in Cell-Line Engineering and Development
Plato wrote in ancient Greece that “our need will be the real creator,” which transformed over time into the English proverb, “Necessity is the mother of invention.” Advancements in medicine and biomanufacturing technology in 2020 have epitomized that idea. Even as technologies such as mRNA vaccines have rocketed into the public’s awareness, biomanufacturing experts have worked behind the scenes with renewed vigor spurred on by hard lessons from the pandemic. Cell-line development and engineering are no exception. Already undergoing a…
Increasing Expression Titers: New Technologies Could Help Other Cell Lines Catch Up to CHO
Fang Tian is a lead scientist and head of cell biology research and development at the American Type Culture Collection (ATCC) in Manassas, VA. She is a member of both the International Cell Line Authentication Committee (ICLAC) and the US technical advisory group for the ISO/TC276 technical committee. At ATCC, she oversees preparation, authentication, characterization, quality control, and cryopreservation of more than 3,400 accessioned animal cell lines and hybridomas in the cell biology general collection. She holds a PhD in…
Engineering Alternatives: Modern Technology Enables Expression System Developers to Think Beyond CHO Cells
Major biopharmaceutical companies are teaming up with academics and the Bill & Melinda Gates Foundation to develop new biomanufacturing cell lines and methods. The project — known as the AltHost Consortium — is exploring innovative ways to produce biologics and vaccines for clinical usage in diseases from diabetes to cancer. Lead researcher J. Christopher Love at the Massachusetts Institute of Technology (MIT) likens this precompetitive, open-access collaboration to the early days of the biopharmaceutical industry. “When biomanufacturing first emerged as…
Cell-Free Expression: A Technology with Truly Disruptive Potential
Bioprocess engineer Beatrice Melinek is a postdoctoral research fellow at University College London’s Future Targeted Healthcare Manufacturing (FTHM) Hub, where she focuses on the use of cell-free protein synthesis (CFPS) as a platform for distributed production of stratified biotherapeutics. Previously Melinek specialized in purification of viral vectors and vaccines, with an engineering doctorate (EngD) in biochemical engineering and postdoctoral experience in UCL’s hematology department developing a new chromatography-based analytical method for measuring empty and full adenoassociated virus (AAV) capsids. She…
Technologies and Innovations: A Discussion with Selexis SA
Pierre-Alain Girod is chief scientific officer (CSO) for Selexis SA. He holds a PhD in plant biochemistry from the University of Lausanne in Switzerland and completed a postdoctoral fellowship at the University of Wisconsin in Madison, WI, on the degradation of proteins by the ubiquitin pathway. Girod returned to Switzerland in 1993, where he discovered a family of sequences that are involved in the epigenetic regulation of genes. That discovery subsequently has been used to express therapeutic proteins in the…
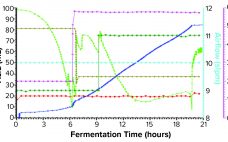
Oxygen Control Strategy and Yield of Recombinant Antibody Fragments Produced in Fermentation
Immunoglobulin molecules are used extensively in therapeutic treatments, diagnostic applications, and fundamental academic research. Traditionally, full-length antibodies and smaller fragments such as the recombinant antigen-binding fragment (rFab) are produced through mammalian cell culture. rFabs also are small enough to be produced in Escherichia coli through fermentation (1, 2). Because disulfide bonds cannot be formed efficiently in the reducing cytoplasm of E. coli, rFabs are supplemented most commonly with a signal sequence that directs them to the more oxidizing bacterial periplasm…
Technology to Transform AAV Manufacture
Adenoassociated virus (AAV) vectors are a popular choice for modern gene therapies because of their favorable safety profile, low immunogenicity, and the ease with which they can be transduced into different cell and tissue types. An AAV genome is a single strand of DNA comprising a replication (rep) gene, which encodes regulatory proteins involved in genome replication, and a capsid (cap) gene, which produces three capsid proteins. However, AAVs cannot replicate alone. In nature, AAV shares an exquisite relationship with…
Optimizing Cell Line Development for High-Quality Biologics
For a host-cell system to generate high yields of recombinant proteins and other entities, cells must be derived from optimized and stable cell lines. However, cell line development (CLD) can be tedious and time-consuming work, and every stage in the CLD workflow has its limitations and challenges. Researchers are creating advanced strategies and tools to overcome those challenges, especially for complex biologics such as bispecific antibodies (BsAbs) and difficult-to-express (DTE) proteins. Online presentations from the CLD track of the BioProcess…