Injectable-drug formulations for both subcutaneous and intravenous administration are designed to be consistent with the number of solutes present in human tissue. Such consistency with physiological conditions is achieved by adding an appropriate amount of salt and/or sugar to attain the desired tonicity. Care must be taken to prevent exposure of cells to either hypotonic or hypertonic formulations that could cause lysis or shrinking, respectively (1). Injection of formulations that deviate from human-plasma osmolality (295 mOsm) can cause pain upon…
Formulation
Formulation, Fill and Finish of Lentiviral Vectors: Part 1 — Case Study in Facility and Process Design
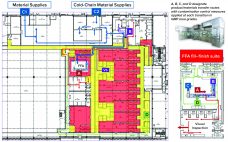
Over the past few years, Oxford Biomedica Ltd. (OXB) has developed and implemented a fill–finish platform (“Oxbox,” Figure 1) at its viral vector processing facility in the United Kingdom. The facility includes four segregated bulk viral-vector drug substance (VS) suites, where closed systems and bioburden control processes apply, and two viral-vector drug product (VP) fill–finish suites that apply aseptic processing, with space for expansion by scale-out as product output demand increases. Segregated suites enable the facility to process different viral…
Spurring on Innovation in Gene Therapy Development
Gene therapies based on adenoassociated virus (AAV) vectors hold promise for treating myriad conditions. Immunogenicity remains a challenge for such products, however. With support from PerkinElmer, Roland W. Herzog (professor of pediatrics and Riley Children’s Foundation professor of immunology at the Indiana University School of Medicine) joined Nagendra Venkata Chemuturi (scientific director of global research for drug metabolism and pharmacokinetics, DMPK, at Takeda Pharmaceuticals) to deliver a BPI “Ask the Expert” presentation exploring strategies for minimizing immune responses to AAV-based…
Aseptic Considerations in Formulation, Fill and Finish: Choosing Between Barrier and Isolator Technologies
Biological drug substances are constituent in a wide range of medicinal products with an even broader spectrum of applications. Those include autoimmune-disease treatments (e.g., for arthritis), vaccines, and recombinant therapeutic proteins (e.g., for cancer treatment). What such products all have in common is that they are manufactured using biotechnology and other cutting-edge technologies. Biologics are not as physically robust as their small-molecule counterparts. Hence, during biomanufacturing processes, these complex molecules present a number of challenges. Some of the typical shared…
Creative Formulation: A Useful Approach to Patient-Centered Drug Development
The development of new medicines is a highly regulated process focused on demonstrating efficacy and safety of new products. Although such qualities always will remain the primary focus of drug development, the biopharmaceutical industry gradually has adopted additional design aspects. New approaches can help meet patients’ divergent needs to improve their lives in meaningful ways. Often referred to as “patient-centered” or “patient-focused” drug-product design, such considerations are expected by many experts to become an increasingly dominant part of future drug…
Analysis of Trace-Level, High-Risk HCPs: Proteomics Advances for Preventing Degradation of Polysorbates in Biotherapeutic Formulations
Polysorbate-80 (PS-80) and polysorbate-20 (PS-20) are used widely in formulation of biotherapeutic products for preventing surface adsorption and as stabilizers against protein aggregation (1). Degradation of polysorbates can cause turbidity and potential formation of subvisible particles mainly consisting of poorly soluble hydrophobic free fatty acids (1). Polysorbate degradation is an industry-wide challenge both in biotherapeutics processing and formulation development. The risk of such degradation increases with higher cell densities and greater expression titers in bioprocessing, as well as with higher…
Single-Use Technology for Formulation and Filling: A Case Study from Swissfillon AG and Pall Corporation
Swissfillon AG is a contract manufacturing organization (CMO) based in Switzerland. Fully compliant with current good manufacturing practice (CGMP) regulations, it provides state-of-the-art aseptic filling for pharmaceutical and biotechnology companies, from clinical-phase materials to commercial quantities. This CMO specializes in high-value, difficult-to-fill products. Swissfillon recognized that adoption of single-use systems (SUS) on a commercial scale required major improvements in consistency and reliability compared to manual operations at pilot and clinical-trial scale. The single-use formulation and filling process, which includes an…
Eradicating the Need for Cold-Chain Distribution in the Biopharmaceutical Industry
Cold chain distribution is complicated and critical for formulations that must be kept in very cold temperatures in the pharmaceutical industry, since their stability decreases quickly at room temperatures. The World Health Organization (WHO) has reported over 50% of vaccines are wasted and must be disposed of globally every year due in part to disruption of the cold chain distribution and lack the resources to support the ultracold temperature requirements. A possible solution to the existing problem is Hyalo Technologies’…
Ask the Expert: Formulation Strategies for AAV-Based Gene Therapies
Although formulation and drug-product activities are critical to developing gene therapies, much remains to be learned about their degradation mechanisms, and firm criteria still need to be established for buffer and excipient selection. Sarathi Vijay Boddapati (associate director of formulation and drug development at Catalent Cell and Gene Therapy) joined BPI on 25 March 2021 to present what his company continues to learn about formulating gene therapies by adapting methods used for other biologics. Boddapati’s Presentation Adenoassociated virus (AAV) remains…
Stability Testing: Monitoring Biological Product Quality Over Time
Many physical and chemical factors can affect the quality, safety, and efficacy of biopharmaceutical products, particularly after long-term storage in a container–closure system that can be subject to variations in temperature and light, as well as agitation with shipping and handling. Proteins are inherently complex physiochemically, from their primary amino acid sequences to their higher-order structures, and they require specific conditions to maintain their integrity and functionality. Advanced biological therapies can be even more complicated and particular about their environments.…