Scalability remains a critically important topic for biopharmaceutical companies. For conventional protein products, the strategy once was straightforward: Drug makers would scale up, beginning with cultures in flasks and roller bottles to grow enough cells to inoculate laboratory-scale (often glass) bioreactors, then again to pilot- and commercial-scale, stainless-steel, stirred-tank bioreactors. At their highest volumes, such reactors can handle tens of thousands of liters of cells and growth media. If a drug developer did not have the requisite equipment to scale…
Upstream Development
Scaling AAV Production: Easing the Transition from Laboratory Scales to Commercial Manufacturing
Adenoassociated virus (AAV) has emerged as the leading vector for gene therapy delivery. Compared with options such as lentivirus and adenovirus, AAV exhibits a strong safety profile because it has low pathogenicity and requires a helper virus to replicate. AAV is also capable of long-term gene expression, and it can infect both dividing and nondividing cells (1–5). Developers of advanced therapies have found such advantages to be quite attractive. As of January 2021, two gene therapy products have gained US…
Mind the Gap: Managing Relationships Between Upstream and Downstream Intensification
Process intensification (PI) describes an integrated framework of strategies to maximize the output of a unit operation, a process, or an entire facility. By implementing PI strategies, biomanufacturers can accomplish their productivity goals by increasing production speeds and titers, reducing facility footprints, and cutting costs. Overall, such changes improve production efficiency and flexibility. Collectively, the biotherapeutic industry has made multiple advancements in intensifying upstream processing. PI strategies include using high-density cell banks, implementing seed-train intensification (n – 1 perfusion), and…
Posttranslational Modifications and Their Control in CHO Culture
The Chinese hamster ovary (CHO) cell line was first established by Theodore Puck in the 1950s and was used mainly for cytogenetics studies (1). Since the 1990s, CHO cells have increased in popularity as expression host cells because they can be adapted easily into suspension culture and genetically modified. The CHO cell line also has a human-like glycosylation profile (2–4). Therapeutic proteins undergo different posttranslational modifications (PTMs) during manufacturing. Some modifications can lead to undesired effects such as decreased stability,…
Accelerating the Development and Manufacture of Therapeutics Using the Octet Platform
The high costs of therapeutic discovery, development, and manufacture require improved process efficiencies and economics. Analytical tools that eliminate the need for reagent labeling and enable real-time data visualization save development time and improve efficiencies during process development. The Octet biolayer interferometry (BLI) platform and assays can be used throughout process development and manufacturing, including cell-line development, clone selection, and dynamic binding capacity (DBC) determination for affinity purification columns. The ability of the Octet BLI platform to monitor binding interactions…
Scalable, Real-Time Bioprocess Monitoring Solution: Kaiser Raman Technology and Sartorius BioPAT Spectro Yield Value from Early Process Development to Single-Use Manufacturing
Raman spectroscopy is used in biomanufacturing as a process analytical technology (PAT) tool for making rapid, nondestructive, in-process measurements. However, Raman data collection at early stages of bioprocess development has been a challenge because of the lack of interface to bioreactors <250 mL. Realizing the industry was struggling to capitalize on the full potential of Raman spectroscopy, Kaiser Optical Systems, Inc. (Kaiser), an Endress+Hauser company, collaborated with Sartorius to bring Raman to Ambr 15 microbioreactors (10–15 mL) and Ambr 250…
Measuring Viral Titer in AAV-Mediated Gene Therapy Using a PCR Technique for Absolute Quantitation
Gene therapies have reemerged as promising treatments to a number of genetic illnesses. Nearly 400 gene therapy clinical trials are recruiting or ongoing in the United States for diseases such as hemophilia and spinal muscular atrophy (1). The primary vehicles used today to deliver such therapies to patients’ cells are viral vectors such as adenoassociated viruses (AAVs), but producing biologically active vectors for gene therapy can be problematic. One difficulty is generating vectors at the correct concentration to yield a…
How Capacitance Measurement Can Improve Viral Vector and Virus-Based Vaccine Production
With the increasing development of viral vector and virus-based vaccines, technologies that help to manufacture and scale up these types of vaccine quickly and cost-effectively have become more critical. By accessing this special report from Aditya Bhat, a capacitance technology expert at Aber Instruments, you will find out how in-line capacitance measurement can produce a detailed fingerprint of cell culture processes and how that can benefit vaccine production. From case studies involving baculovirus, AAV, and measles, you will discover why…
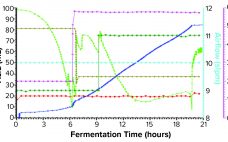
Oxygen Control Strategy and Yield of Recombinant Antibody Fragments Produced in Fermentation
Immunoglobulin molecules are used extensively in therapeutic treatments, diagnostic applications, and fundamental academic research. Traditionally, full-length antibodies and smaller fragments such as the recombinant antigen-binding fragment (rFab) are produced through mammalian cell culture. rFabs also are small enough to be produced in Escherichia coli through fermentation (1, 2). Because disulfide bonds cannot be formed efficiently in the reducing cytoplasm of E. coli, rFabs are supplemented most commonly with a signal sequence that directs them to the more oxidizing bacterial periplasm…
The Upstream Perspective: Taking Efficiency Beyond Cell-Line Development
With 20 years of experience in the biopharmaceutical industry — at Genentech, Applied Biosystems, Cell Genesys, Cellerant Therapeutics, and Bayer — Yuval Shimoni has written frequently for BioProcess International on a number of production topics. Those have ranged from process improvements and bioreactor scale-down validation, to raw materials management, to addressing variability and virus contamination events. For this featured report, we discussed hardware and instrumentation, quality by design (QbD) and related approaches, and other strategies that can take expediting upstream…