The commercial manufacturing success of monoclonal antibodies (MAbs) has become a touchstone of the biopharmaceutical industry. MAbs are so well established that they often are referred to as “traditional” biologics, and well-known MAb processing methods have become a model for processing of other “advanced” or “emerging” therapies. But MAb processing continues to advance as biomanufacturers seek ways to improve efficiencies, lower costs, and (most recently) increase sustainability of facilities. Drug makers are particularly interested in strategies for MAb process intensification.…
MAb
Antibody-Derivative Biotherapeutics: Fragments and Fusions Define the Future
Monoclonal antibodies (MAbs) remain the dominant biopharmaceutical product class, but as biotechnologies have advanced in recent decades, developers have found ways to exploit their “magic-bullet” capabilities while putting aside their limitations. That has led to a new generation of antibody-related therapeutics created by cutting and pasting molecular domains. Researchers are mixing and matching functional moieties of antibodies and other molecules to create custom-designed proteins with powerful efficacy and tunable targeting. A simple search at Taylor & Francis Online (https://www.tandfonline.com), the…
Development and Manufacture of Therapeutic Bispecific Antibodies
To meet the ongoing need for new and improved drugs, the biopharmaceutical community strives to create molecules with new functions. Bispecific antibodies (bsAbs), which can simultaneously home in on two different targets, illustrate the scientific ingenuity needed for this task. The basic proof of concept for these complex molecules was established in 1960 (1), and their application to the redirection of effector cells was reported in the mid-1980s (2–4), but producing them has proved to be challenging. Many technical advances,…
Expression of Recombinant Antibody Fragments: High-Density Fermentation in Multiuse and Single-Use Systems
Single-use fermentors (SUFs) offer dramatic advantages over traditional stainless-steel clean-in-place (CIP)/sterilization-in-place (SIP) fermentors. Single-use technology eliminates the need for steam, chemicals, and water to clean and sterilize stainless vessels, which provides a direct reduction in capital cost and environmental-impact mitigation. Single-use platforms also increase equipment and process flexibility significantly, making it possible to switch campaigns from one product to another in minimal time, while eliminating cleaning and associated validation steps (1). Both Cytiva and Thermo Fisher Scientific offer SUFs that…
Lymphocyte Activation Gene 3 in Immunooncology: A Soluble Protein Alternative
Early in 2021 at the American Society of Clinical Oncology’s (ASCO’s) annual meeting, attendees witnessed the first validation of a novel checkpoint target: lymphocyte activation gene 3 (LAG-3). Bristol Myers Squibb’s (BMS’s) recent success in a phase 3 study of the relatlimab anti-LAG-3 monoclonal antibody (MAb) proved that the combination of LAG-3 and programmed cell death protein 1 (PD-1) is more effective than the standard of care in first-line metastatic melanoma (1). For about seven years, that standard has been…
eBook: Bispecific Antibodies — Their Development and Manufacture As Therapeutics
Generating antibodies with two or more specificities is one of the most innovative fields in therapeutic antibody development, with tremendous potential for use in creating new treatments for patients with unmet medical needs. In particular, bispecific antibody development is stimulating innovations in bioprocessing techniques from expression through upstream processing and candidate purification. Wherever possible, process-development scientists and engineers are borrowing techniques that were honed for mature monoclonal antibody (MAb) platforms, then applying those to bispecific antibody manufacturing. Nevertheless, the unique…
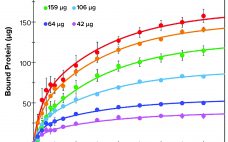
Evaluation of a Novel Peptide-Based Affinity Ligand for Human IgM Purification: Use of an Automated Liquid-Handling System for Rapid Assessment of Binding Kinetics and Capacity
One-step affinity purification of antibodies is a powerful and widely used tool in the biopharmaceutical industry. Although different strategies can be used to purify immunoglobulin isotype G (IgG), the larger antibody isotype IgM has limited options. Human IgM is the largest antibody and primarily exists as a pentamer in the bloodstream (1). IgM molecules exhibit higher avidity than other antibodies do and serve as essential activators for the complement cascade (1–3). Approaches to IgM purification with hydroxyapatite and ion-exchange (IEX)…
Regeneron confident it can scale-up to meet US COVID MAb demands
Regeneron has entered an agreement worth $2.625 billion with the US government and believes it can supply 1.25 million additional doses of its monoclonal antibody cocktail for COVID-19.  The US Department of Health and Human Services (HHS)and the Department of Defense (DOD) will purchase finished doses of the antibody cocktail, REGN-COV2, a combination of casirivimab and imdevimab by June as part of Operation Warp Speed (OWS). The new agreement comes after the Biomedical Advanced Research and Development Authority (BARDA) granted Regeneron…
Making MAbs: Bioprocess Advancements Challenge Platform Assumptions
Still the largest sector of the industry, monoclonal antibodies (MAbs) have dominated the biopharmaceutical stage for over 30 years. Some observers might think there’s nothing new to say about these molecules; others point to antibody derivatives as a more exciting alternative. But MAbs are far from an outdated technology. From biosimilar developments to cell-free synthesis to yeast display, immunogenicity improvements, and fully human antibodies — as well as improvements in process efficiency and cost reductions, as discussed herein — the…
Tracking Therapeutic Antibody Development in a Pandemic
The COVID-19 pandemic has generated a significant and rapid response from scientists who aim to develop drugs and vaccines in the academic, government, and industrial sectors. Such interventions are essential to containing SARS-CoV-2, the coronavirus that causes the COVID-19 disease. To inform and educate the public about global efforts to develop targeted therapies such as monoclonal antibodies (MAbs), The Antibody Society (TAS) and the Chinese Antibody Society (CAS) have designed and implemented a freely available online database called the COVID-19…