Even in “normal” times, companies need to balance time to filing an investigational new drug (IND) application against careful consideration of processes that can have far-reaching consequences on the quality of biologic products. But supply-chain interruptions still feature prominently in the news during the ongoing COVID-19 pandemic. From equipment to chemicals to plastic components, end users, their suppliers, and (critically) their suppliers’ suppliers all are feeling growing uncertainty about production timelines and availability of materials. In this eBook, BPI’s editor…
Author Archives: Susan Dana Jones
eBook: Speed to IND — Balancing Risk and Reward
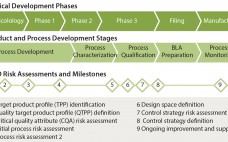
With so many biopharmaceuticals obtaining breakthrough or fast-track designations, companies that use accelerated strategies to be first in human studies can be left with significant quality and manufacturing challenges that must be solved later on. Despite regulatory encouragement to create solid design spaces and define parameters according to quality by design (QbD), those may go by the wayside given the pressures of speed. The reward is the investigational new drug (IND) application itself — but if companies lock in subpar…
eBook: Quality By Design for Monoclonal Antibodies — Establishing the Foundations for Process Development, Design Space, and Process Control Strategies
The quality by design (QbD) modernized approach to pharmaceutical development is intended to provide regulatory flexibility, increased development and manufacturing efficiency, and greater room to innovate as well as improve manufacturing processes within defined ranges without obtaining regulatory approval first. QbD is a systematic developmental approach that starts with a clear goal in mind and emphasizes understanding of how variability in both process and materials affects a final product (1). Historically, product quality has been assured either with end-product testing…
Quality By Design for Monoclonal Antibodies, Part 2: Process Design Space and Control Strategies
Process design space and control strategy are two fundamental elements of quality by design (QbD) that must be established as part of biopharmaceutical development and regulatory filings. Like all of QbD, they are interconnected and iterative. Both are based on knowledge gained during product and process development — but both need to be in place (in a potentially very limited form) when a company begins to manufacture drug substance for clinical trials. Part 1 of this discussion appears on pages…
Quality By Design for Monoclonal Antibodies, Part 1: Establishing the Foundations for Process Development
The quality by design (QbD) modernized approach to pharmaceutical development is intended to provide regulatory flexibility, increased development and manufacturing efficiency, and greater room to innovate as well as improve manufacturing processes within defined ranges without obtaining regulatory approval first. QbD is a systematic developmental approach that starts with a clear goal in mind and emphasizes understanding of how variability in both process and materials affects a final product (1). Historically, product quality has been assured either with end-product testing…
From CMO to CDMO: Opportunities for Specializing and Innovation
Biopharmaceutical contract manufacturing organizations (CMOs) were initially enabled when the requirement for a company to file for both an establishment license application and a product licensing application transitioned to the current format of a biologics license application (BLA) submission for biological products (1). The initial focus of such CMOs was to provide large-scale, commercial manufacturing for companies that had already developed and validated bio manufacturing processes. Consequently, CMOs were generally formed as stand-alone service providers that “rented” manufacturing capacity to…
Single-Use Technology and Modular Construction
To enable broad, global access to life-saving biopharmaceutical products, our industry is facing significant pressure to reduce the overall cost of manufacturing and enable local manufacturing where possible. Combined with growing markets outside the United States and Europe and development of high-titer, high-yield processes, that pressure has led to a shift in the industry’s approach to facility design and construction. Today’s biopharmaceutical production facilities must be flexible, cost effective, and readily constructed with minimal capital investment and construction timelines. As…
Efficient, Flexible Facilities for the 21st Century
A number of recent improvements in the engineering of high-titer expression vectors, in biopharmaceutical process development, and in facility construction have converged to present new opportunities for cost-effective, flexible, biomanufacturing facility construction. The evolution of requirements for biopharmaceutical facilities is driven by globalization of the biopharmaceutical industry, patent expirations of several blockbuster biopharmaceutical products, and the increasing shift in new product development away from blockbuster drugs and toward more personalized, niche products. An increase in product approvals (primarily…
Emerging Challenges in Cell Therapy Manufacturing
The introduction of recombinant proteins and monoclonal antibody (MAb) products revolutionized the treatment of many diseases, including diabetes, rheumatoid arthritis, multiple sclerosis, Crohn’s disease, cardiac disease, and cancer. These highly specific biologic therapies provide patients with life- saving approaches that are not possible with small molecules. MAbs in particular are a unique class of biopharmaceutical products that interact with and activate components of the immune system to provide such therapeutic benefits as tumor destruction by antibody-dependent cell-mediated cytotoxicity…
Ongoing Challenges of Applying QbD to Biopharmaceutical Products
Quality by design (QbD) was a hot topic at IBC’s BioProcess International Conference and Exhibition, 20–24 September 2010 (Providence, RI). For her keynote address, Helen Winkle (director of the FDA’s Office of Pharmaceutical Science) discussed the agency’s continuing efforts to improve product quality regulation as well as opportunities and challenges of implementing QbD for biotechnology products (1). Since introducing its 21st Century Initiative in 2002, the FDA has made some headway toward enhancing product quality through QbD (2). The QbD…