Antithrombin alfa is a recombinant human antithrombin developed as an anticoagulant treatment for people with hereditary antithrombin deficiency who are undergoing surgical or childbirth procedures (1). Marketed under the ATryn brand name by LFB SA (Les Ulis, France), antithrombin alfa was approved for use in adults by the US Food and Drug Administration (FDA) in February 2009 (2). Antithrombin alfa is expressed in the milk of transgenic goats and purified through a multistep downstream process encompassing both filtration and chromatography.…
Downstream Validation
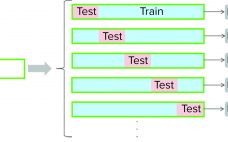
Multivariate Data-Driven Modeling for Continued Process Verification
Continued process verification (CPV) is an integral part of process validation for the manufacture of human and animal drugs and biological products (1). It is designed to meet three primary goals: maintain a validated state of products, their processes, and related systems; enable continuous process improvements; and meet regulatory requirements for life-cycle validation. A CPV program for a biologic product entails regular collection of data related to critical process parameters (CPPs) and critical quality attributes (CQAs) and the preprocessing, analysis,…
Risk Determination of Potential Mycotoxin Exposure to Patients: Testing Recombinant Human Factor VII from Transgenic Rabbits
Sevenfact eptacog beta is a new recombinant human factor VIIa (rFVIIa) developed by LFB SA in Les Ulis, France, as a bypassing agent (BPA) for treatment and control of bleeding in people with hemophilia A and B and inhibitors (1, 2). The product was approved for use in adults and adolescents by the US Food and Drug Administration (FDA) in April 2020 (3). It is expressed in the milk of transgenic rabbits and purified through a multistep process using both…
eBook: Process-Related Impurities — Emerging Strategies for Detection, Identification, and Management of Host-Cell Proteins
Host-cell proteins (HCPs) represent a major class of process-related impurities (PRIs) that are generated during biopharmaceutical manufacture. Although the vast majority of such proteins are removed from a drug substance during downstream purification, residual HCPs can remain in a finished drug product. Even in minimal concentrations, copurifying HCPs can pose safety risks and compromise protein-product yield, efficacy, and stability. Thus, regulatory agencies consider the presence of HCPs to be a critical quality attribute (CQA). Sufficient clearance of these impurities helps…
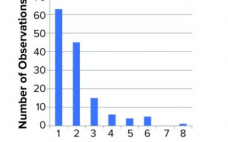
Virus Assay Variation Is the Main Source of Variation in Viral Clearance Studies: Retrospective Analysis of a Large Data Set
Biopharmaceuticals produced from mammalian cell cultures are susceptible to viral contamination. That risk is mitigated by applying complementary approaches. Those include extensive testing of cell banks, selecting low-risk raw materials, testing cultivations for viruses, and documenting the capacity of a purification process to inactivate and remove viral contaminants. The latter commonly is referred to as viral clearance and usually expressed as a log reduction value (LRV). Novo Nordisk has performed several viral clearance studies for different processes and process steps.…
HCP Assay Development: Managing Risks with Evolving Technologies
Host-cell proteins (HCPs) are major impurities of concern in biomanufacturing. When present in drug formulations, they can reduce efficacy (by compromising product stability), introduce toxicity, and increase a recipient’s risk for long-term immunogenicity. Understanding HCP profiles and integrating effective removal strategies are important parts of developing new biological drugs — to fulfill regulatory guidelines and to ensure patient safety through product quality. HCP populations can be both complex and structurally diverse, and some changes in upstream culture conditions can affect…
Emerging Strategies for Drug Product Comparability and Process Validation: Part 1 — Analytical Tools and Drug Product Comparability
Process validation is a key part of the development and manufacture of all approved drug products, but its completion can be a daunting task. At a two-day CASSS CMC Strategy Forum held in July 2016 in Gaithersburg, MD, speakers and attendees addressed the many technical, practical, and regulatory facets of drug product process validation and comparability. In part 1 of this report, we summarize the key discussion points of the first day, which focused on analytics and comparability. Session One:…
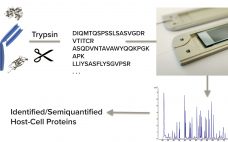
Nontargeted HCP Monitoring in Downstream Process Samples: Combining Micro Pillar Array Columns with Mass Spectrometry
Protein biopharmaceuticals have emerged as important treatments for diseases with otherwise unmet medical needs. These biologics are produced by recombinant mammalian, yeast, or bacterial expression systems. Along with therapeutic proteins, those cells produce endogenous host-cell proteins (HCPs) that can contaminate biopharmaceutical products despite multiple purification steps in downstream processing. Because such process-related impurities can affect product safety and efficacy, they need to be monitored closely. Multicomponent enzyme-lined immunosorbent assays (ELISAs) presently are the workhorse method for HCP testing, with high…
Breaking the Bottlenecks: Accelerating Viral Vector Bioanalysis in Downstream Processing
The rise in popularity of viral-vector–based gene therapies and SARS-CoV-2 vaccines has created a shortage of manufacturing capacity, driving efficiency improvements to avoid bottlenecks in bioanalysis to support culture optimization and bioprocessing steps. To improve workflows in bioanalytical assay development and sample analysis, more efficient methods are needed that include higher throughput, simpler and more streamlined sample assay methods, wide dynamic ranges, and efficient data processing and interpretation. Gyrolab™ microfluidic, nanoliter-scale immunoassay systems provide an innovative, robust solution to high-performance…
Bioassay Evolution: Finding Best Practices for Biopharmaceutical Quality Systems
Bioassays help drug developers determine the biological activity (potency) of their products, which has been a biopharmaceutical critical quality attribute (CQA) since long before that concept had a name. Because of their complex nature, bioassays are among of the most challenging experiments to perform reliably with dependably accurate results. Consistent assay performance requires a controlled environment and qualified reagents; skilled analysts who understand cell physiology, regulatory requirements, and the latest techniques; and assay protocols that are intelligently developed, characterized, and…