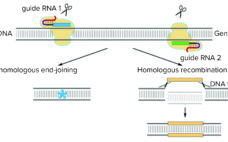
Since January 2016 (with a brief interlude as described below), the Patent Trial and Appeal Board has been attempting to adjudicate the proper inventorship of CRISPR technology in (to date) six separate patent-interference proceedings. (Scientific “priority has been decided, for now, by the awarding of the Nobel Prize in Chemistry to Jennifer Doudna and Emmanuelle Charpentier in 2020; see the “Priority Claims” box). CRISPR-based gene editing was hailed as the “Breakthrough of the Year” in 2015 (1), and the scientific…
Emerging Therapeutics
Antibody-Derivative Biotherapeutics: Fragments and Fusions Define the Future
Monoclonal antibodies (MAbs) remain the dominant biopharmaceutical product class, but as biotechnologies have advanced in recent decades, developers have found ways to exploit their “magic-bullet” capabilities while putting aside their limitations. That has led to a new generation of antibody-related therapeutics created by cutting and pasting molecular domains. Researchers are mixing and matching functional moieties of antibodies and other molecules to create custom-designed proteins with powerful efficacy and tunable targeting. A simple search at Taylor & Francis Online (https://www.tandfonline.com), the…
Development and Manufacture of Therapeutic Bispecific Antibodies
To meet the ongoing need for new and improved drugs, the biopharmaceutical community strives to create molecules with new functions. Bispecific antibodies (bsAbs), which can simultaneously home in on two different targets, illustrate the scientific ingenuity needed for this task. The basic proof of concept for these complex molecules was established in 1960 (1), and their application to the redirection of effector cells was reported in the mid-1980s (2–4), but producing them has proved to be challenging. Many technical advances,…
Expression of Recombinant Antibody Fragments: High-Density Fermentation in Multiuse and Single-Use Systems
Single-use fermentors (SUFs) offer dramatic advantages over traditional stainless-steel clean-in-place (CIP)/sterilization-in-place (SIP) fermentors. Single-use technology eliminates the need for steam, chemicals, and water to clean and sterilize stainless vessels, which provides a direct reduction in capital cost and environmental-impact mitigation. Single-use platforms also increase equipment and process flexibility significantly, making it possible to switch campaigns from one product to another in minimal time, while eliminating cleaning and associated validation steps (1). Both Cytiva and Thermo Fisher Scientific offer SUFs that…
Lymphocyte Activation Gene 3 in Immunooncology: A Soluble Protein Alternative
Early in 2021 at the American Society of Clinical Oncology’s (ASCO’s) annual meeting, attendees witnessed the first validation of a novel checkpoint target: lymphocyte activation gene 3 (LAG-3). Bristol Myers Squibb’s (BMS’s) recent success in a phase 3 study of the relatlimab anti-LAG-3 monoclonal antibody (MAb) proved that the combination of LAG-3 and programmed cell death protein 1 (PD-1) is more effective than the standard of care in first-line metastatic melanoma (1). For about seven years, that standard has been…
eBook: Antibody–Drug Conjugates —
A New Generation of Approaches Is Changing the Game
Combining large proteins with linkers and cytotoxins, antibody–drug conjugates (ADCs) may be the most complex drug molecules in development today. Despite early promise and product approvals, a number of technical concerns arose during product and process development. Characterizing and ensuring consistency in the number of small molecules that attach to the antibody — as well as ensuring their proper attachment and biophysics — all present significant challenges to ADC developers. Solving early problems associated with product quality has introduced a…
Chromatography in mRNA Production Workflow
Rapid response to global pandemics requires the manufacture of billions of vaccine doses within months. This short timeline must allow for design and testing of active ingredients, development of production and purification processes, clinical evaluations, regulatory filings, and manufacturing. Existing purification methods often have been adopted from laboratory-scale techniques to allow rapid implementation, and those have provided adequate product quality. But future mRNA development will require optimized production and purification processes. Chromatography has been a workhorse of biomanufacturing for decades,…
eBook: Rare Diseases — Biopharmaceutical Challenges Presented By Relatively Small Patient Populations
By definition, an orphan disease affects a small percentage of the population. However, with some 7,000 such conditions identified so far, they collectively have a significant impact on global health. An estimated 350 million people are affected worldwide by a rare disease — altogether more than the population of the world’s third largest country (the United States). Some well-known biopharmaceutical companies are devoted to developing treatments for rare diseases. However, the vast majority of such diseases have no treatments approved by…
Microbiome-Based Therapeutics: Negotiating Key Development Challenges
Microbiome-based therapeutics have evolved significantly in recent years, with several promising candidates advancing through the clinical pipeline. That progress is the result of growing evidence showing that targeting and manipulating the microbiome could improve human health and treat more than 25 conditions by restoring healthy bacterial populations (1). The human microbiome comprises a diverse community of microorganisms, helpful and harmful. It differs by person based on factors such as genetics, environmental influences, diet, and immune function. Microorganisms play a role…
eBook: Bispecific Antibodies — Their Development and Manufacture As Therapeutics
Generating antibodies with two or more specificities is one of the most innovative fields in therapeutic antibody development, with tremendous potential for use in creating new treatments for patients with unmet medical needs. In particular, bispecific antibody development is stimulating innovations in bioprocessing techniques from expression through upstream processing and candidate purification. Wherever possible, process-development scientists and engineers are borrowing techniques that were honed for mature monoclonal antibody (MAb) platforms, then applying those to bispecific antibody manufacturing. Nevertheless, the unique…