Viral clearance is a fundamental aspect of viral safety for biopharmaceutical products. Regulatory agencies around the world require biomanufacturers to segregate their operations appropriately to mitigate the risks of carryover contamination from previous process steps or product batches and of crossover contamination between product(s) made in the same facility. Guidelines are vague in defining “appropriate,” leaving biomanufacturers to interpret regulatory expectations and define their own virus reduction and segregation strategies. Given the differences among manufacturing processes and facilities housing such…
Downstream Processing
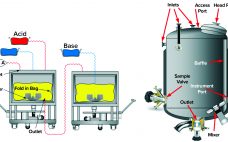
Dye-Stripping Buffer and Resin Stripped-Dye Analysis: Development and Optimization of a Novel Spectrophotometric Assay and Method for Removal of Cibacron Blue Dye
Biopharmaceutical process-related impurities encompass all organic and inorganic materials that arise from the biomanufacturing process apart from the drug substance. According to ICH Q6B guidelines on test procedures and acceptance criteria for biotechnological products, these impurities include cell substrates such as host-cell proteins, host-cell DNA, endotoxins, and so on; inducers, antibiotics, media components, and chromatographic media used in purification processes; and solvents and buffer components (1). Impurities can cause undesired, deleterious pharmacological effects if ingested or injected by a patient,…
Streamlined Polishing and Viral Clearance Using a New Hybrid, Biomimetic, Single-Use Anion Exchanger
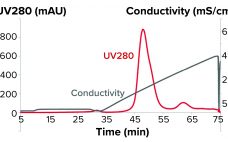
Flow-through anion-exchange (AEX) chromatography is used frequently in biopharmaceutical purification processes for reduction of net–negatively charged host-cell proteins (HCPs) and viruses as part of a validated viral clearance strategy (1, 2). AEX column chromatography is the technology most often used for electrostatic viral clearance, particularly in commercial-scale biopharmaceutical manufacturing, for which columns have a long-established history of reliable and well-understood performance (3). Still, validation of HCP and viral clearance by AEX columns in biopharmaceutical processes involves complexities that contribute significantly…
Simple and Effective Method for Purification of DMT-On Oligonucleotides Using HIC Resins
Within the biopharmaceutical industry, oligonucleotide drug pipelines have increased significantly because of the effectiveness of such drug products in treating devastating diseases. Downstream specialists need improved purification techniques for such highly valuable materials. Dimethoxytrityl (DMT) is used to synthesize oligonucleotides and temporarily mask the characteristic chemistry of the 5Í´-hydroxy functional group. DMT can be left on an oligonucleotide following synthesis to provide stability to a molecule during subsequent processing. Herein, we describe a novel, effective, and high-recovery method for purifying…
eBook: Mixed-Mode Chromatography for Purification of Biopharmaceuticals
Mixed-mode chromatography offers several advantages in downstream processing of biotherapeutics. Mixed-mode chromatography resins use ligands that are capable of at least two modes of interaction with solutes such as hydrophobic, ion exchange, and metal affinity. The interactions between stationary and mobile phases that result from those combinations enhance chromatographic selectivity, facilitating separation efficiencies that are not possible using other chromatography media. As this eBook illustrates, the multimodal approach can save developers time and money by enabling robust purification of biopharmaceuticals…
eBook: Viral Vector Purification — A Discussion of Current Challenges and Methods
Adenoassociated viral (AAV) vectors have become synonymous with gene therapy delivery. However, because they are produced in such small quantities and because their upstream processes carry comparatively large amounts of host-cell DNA and other impurities, AAV purification can be challenging. Several researchers have applied different chromatographic strategies, but no universal method has been adopted in the biopharmaceutical industry. This eBook features a discussion among several industry experts that explores challenges specific to AAV purification, shedding light on whether current strategies…
The Downstream Perspective: Putting Product Knowledge to Work Using Technological Innovations
After over a quarter century in the industry — including downstream processing (DSP) and manufacturing directorships at Boehringer Ingelheim and leadership roles in technology development, quality, and manufacturing at Novasep — European consultant Margit Holzer is a recognized expert in downstream processing of biopharmaceutical products. Holding a doctorate in biotechnology from the University of Natural Resources and Applied Life Sciences in Austria, Holzer is familiar to BPI readers as both an author and conference participant (1, 2). And in May…
Discover, Develop, Deliver
Astrea Bioseparations is the only adsorbent supplier that can discover new affinity ligands designed to bind selectively to a molecule of interest or specific impurity, develop efficient purification adsorbents and downstream methods, and deliver industrial-scale adsorbents (up to 1,000-L batch sizes) as loose slurry or in good manufacturing practice (GMP)-ready columns. With over 30 years of experience in development of affinity products and design and manufacture of new custom adsorbents, Astrea Bioseparations is a world leader in its field. The…
Reduce Downstream Processing Costs for MAbs By Switching to a Two-Step Platform
Downstream processing operations make up to 80% of the total costs for processing biotherapeutics. Given the current drive to reduce downstream costs, chromatographers and process engineers will need to streamline processes. Herein, we describe the benefits offered by using Tosoh’s two-step process for purifying monoclonal antibodies (MAbs) and compare that method with the standard industrial process. By combining high-performance protein A capture and a single polishing step on salt-tolerant anion-exchange resin, Tosoh’s approach can reduce downstream costs by 45% and…
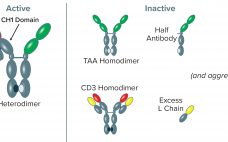
Capture of CH1-Containing Bispecific Antibodies: Evaluating an Alternative to Protein A
Bispecific antibodies (BsAbs) are designed to recognize and bind two different antigens, in many cases for the purpose of immune effector-cell activation to destroy cancer cells (1). Such BsAbs mediate cell killing by binding simultaneously to an antigen that is overexpressed on tumor cells and to the CD3 receptor, activating cytotoxic T lymphocytes (2). Using proprietary UniRat human heavy-chain technology combined with OmniFlic human fixed–light-chain antibody technology licensed from Ligand Pharmaceuticals, Teneobio has produced several bispecific antibodies, each targeting a…