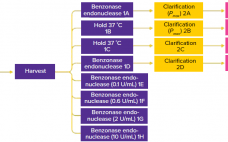
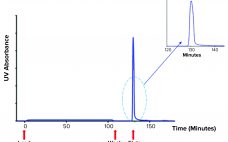
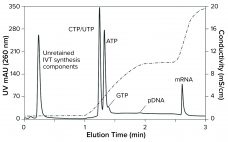
During production of vaccines and viral vectors, the size and quantity of extracellular nucleic acids must be reduced using endonuclease enzymes. Merck/MilliporeSigma’s proprietary Benzonase endonuclease is a genetically engineered nuclease derived from the Gram-negative bacteria Serratia marcescens. It attacks and degrades all forms of DNA and RNA. It is manufactured under good manufacturing practice (GMP) conditions and has a drug master file (MDF) in place with the US Food and Drug Administration (FDA), which can be cited in regulatory filings.…
Downstream Processing
New Antibody Formats on the Block:
More Complex Modalities Demand Innovation in Manufacturing and Purification
Some of the latest, most promising therapeutic developments in the biologics industry use antibody fragments — either separate functional subunits of antibodies or recombinant molecules that are composed of immunoglobulin domains. The most popular fragments are antigen-binding fragments (Fabs), variable single-chain fragments (scFvs), diabodies, and nanobodies. Such molecules raise several advantages over their parent molecules for upstream production but pose several challenges for downstream purification. To facilitate antibody-fragment capture, Tosoh Bioscience has developed Toyopearl AF-rProtein L-650F resin. Its ligand uses…
Ligand-Based Exosome Affinity Purification: A Scalable Solution to Extracellular Vesicle Downstream Bottlenecks
Novel therapeutics based on extracellular vesicles (EVs) recently passed a critical development milestone. During 2020, some of the first experimental EV products developed by biopharmaceutical companies entered human clinical trials (1–3). EVs are nanometer-sized, lipid-wrapped spheres released by almost every cell type in the human body. EVs are loaded with a cargo of proteins, lipids, and RNA, and they are tagged with surface markers that favor uptake by target cells. Thus, EVs are a key mode of cell-to-cell communication (4).…
Eliminating the Analytical Bottleneck in Production and Purification of mRNA
COVID-19 has focused a spotlight on the ability of mRNA technology to accelerate vaccine development and approval (1). That same technology can hasten development and approval of other therapeutic classes, including cancer immunotherapy, protein replacement, and gene therapy. Fulfilling those opportunities imposes significant challenges on process developers and manufacturers to improve existing processes. Scale-up to produce millions of doses (tens of kilograms) compounds those challenges. Furthermore, every step of the journey requires high-performance analytical methods, to ensure patient safety and…
Ask the Expert: Selecting the Right Buffer Management Strategy
Although buffers are among the simplest materials used in bioprocessing, they are critical to biopharmaceutical manufacturing success. Buffer preparation, storage, and handling can require significant investments in time, labor, equipment, and facility space. Jenny Dunker, MSc, and Alexander Troken, PhD (global product managers for customized bioprocess solutions and for process liquids and buffers, respectively, at Cytiva), delivered an “Ask the Expert” presentation on 30 March 2021 to explore strategies for intensifying buffer management. Available Options Biopharmaceutical manufacturers often prepare buffers…
Predicting Viral Clearance in Downstream Process Development
As viruses can arise during the manufacture of biopharmaceuticals, regulatory agencies require viral clearance validation studies for each biopharmaceutical prior to approval. These studies are typically conducted in biosafety level (BSL)-2 facilities and require large capital and human resources. The use of an accurate, economical, and quantifiable noninfectious viral surrogate would enable downstream purification scientists to study viral clearance throughout process development. This report explores the use of a BSL-1 compatible, noninfectious MVM particles to predict viral clearance results over…
eBook: Chromatography Resins — Addressing Challenges in Biologic Purification Workflows
Although chromatography remains the backbone of downstream workflows, selecting appropriate technologies to optimize processes can be challenging. Numerous resin options are available, and the fact that most biologics are large, complex, and inherently unstable further complicates development of a robust workflow. Because chromatography processes can alter a biologic in ways that could impair its intended therapeutic function, investment in process development is critical. Techniques such as design of experiments (DoE) can be used to identify the best approach during process…
Viral Clearance in a Downstream AAV Process: Case Study Using a Model Virus Panel and a Noninfectious Surrogate
Over the past decade, adenoassociated virus (AAV) vectors have become established as leading gene-delivery vehicles. In 2017, the pipeline for gene therapies included 351 drugs in clinical trials and 316 in preclinical development (1–4). As those candidates advance, significant efforts are being made in process development and manufacturing for viral vectors, with the overall goal of reducing process impurities while maintaining the highest possible process yield. To address that goal, industry suppliers have developed innovative AAV-specific separation technologies. Thermo Fisher…
eBook: Buffers — Navigating New Demands on Downstream Raw Materials
Bioreactor titers for monoclonal antibody (MAb) processes have increased significantly since the dawn of the biopharmaceutical industry, yet such gains have instigated bottlenecks for critical high-volume raw materials used in downstream processing, such as buffer solutions. As downstream purification is required for most, if not all, biopharmaceutical products, buffers and their preparation are topics that concern nearly every drug company. But those topics rarely receive direct attention. This BPI eBook explores what factors prompted the current buffer bottleneck and what…
How to Improve the Capturing of Antibody Fragments
Some of the latest promising biopharmaceutical drug substances are antibody fragments. Antibody fragments are either separate functional subunits of antibodies or recombinant molecules, which, just like antibodies, are composed of immunoglobulin domains. These drugs offer several therapeutic advantages over conventional monoclonal antibodies. Upstream processing for antibody fragments is easier than it is for standard antibodies. Recombinant-based antibody fragments can be modified to meet specific needs of affinity, avidity, valence, and action mode. They also can be produced in prokaryotic cells…