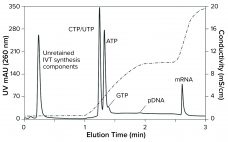
COVID-19 has focused a spotlight on the ability of mRNA technology to accelerate vaccine development and approval (1). That same technology can hasten development and approval of other therapeutic classes, including cancer immunotherapy, protein replacement, and gene therapy. Fulfilling those opportunities imposes significant challenges on process developers and manufacturers to improve existing processes. Scale-up to produce millions of doses (tens of kilograms) compounds those challenges. Furthermore, every step of the journey requires high-performance analytical methods, to ensure patient safety and…
Author Archives: Pete Gagnon
Streamlining Industrial Purification of Adeno-Associated Virus
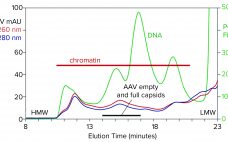
With its first licensed therapeutic now marketed worldwide (1), adeno-associated virus (AAV) has become a preferred vector for gene therapy. However, unlocking its full potential still poses challenges, many of which are associated with purification. The first involves the transition from upstream to downstream processes. AAV-bearing lysates are laden with debris that foul filtration media and limit or prevent concentration. Another challenge involves reduction of soluble host-cell DNA, which is complicated by its strong association with nucleoproteins. A third involves…
A New Runway for Purification of Messenger RNA
A high-performing capture method is a critical bedrock asset for developing industrial purification processes. This is especially true for extended families of products that share highly similar chemical composition. Therapeutic monoclonal IgG is an example. The ability of protein A affinity chromatography to achieve 95% purity in one simple step was the runway that got recombinant immunotherapy off the ground and made it available to millions. In fact, protein A did more. Beyond giving the industry a foundation manufacturing method,…
eBook: Viral Vector Purification — A Discussion of Current Challenges and Methods
Adenoassociated viral (AAV) vectors have become synonymous with gene therapy delivery. However, because they are produced in such small quantities and because their upstream processes carry comparatively large amounts of host-cell DNA and other impurities, AAV purification can be challenging. Several researchers have applied different chromatographic strategies, but no universal method has been adopted in the biopharmaceutical industry. This eBook features a discussion among several industry experts that explores challenges specific to AAV purification, shedding light on whether current strategies…
eBook: Challenges in Industrial Process Development of Exosome-Based Therapies: Characterizing and Managing Diversity
The traditional classification of extracellular vesicles (EVs) includes three types: exosomes, microvesicles, and apoptotic vesicles. Each type arises from a distinct origin and exhibits distinct characteristics. The problem is that their size ranges overlap and that the major surface proteins presented by exosomes also are present on the surfaces of microvesicles and apoptotic bodies. This makes it a challenge for process developers to identify the vesicle fraction that best serves a particular exosome therapy. Anion-exchange chromatography (AEC) can fractionate EVs…
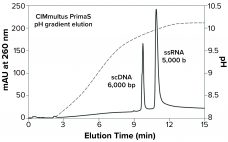
CIMmultus PrimaS: The Next-Generation Anion Exchanger for Today’s mRNA-Based Vaccines
CIMmultus PrimaS multimode ligand bioprocessing technology represents a new class of anion exchangers for fast, efficient, one-step purification of single-stranded mRNA at ambient temperature. DNA, dsRNA, and proteins are eliminated with a high-salt wash. Then ssRNA is fractionated by size in a pH gradient that removes short transcripts and fragments. Convective mass transfer and laminar flow through the technology’s monolithic architecture maintain high capacity, high resolution, and low shear stress — even at flow rates >5 column volumes per minute.…
Setting a Cornerstone for Platform Purification of Exosomes
Exosomes are a subject of rapidly growing therapeutic interest in the biopharmaceutical industry for two principal reasons. The first reason is that they are the primary communicators of instructions from source cells to target cells. Exosome surface features define their destination. They recognize complementary features on target cells, dock with them, and deliver their programmed instructions in the form of microRNA. The second reason is that exosomes are immunologically silent. As normal human cell products, and by contrast with gene…
Platform Purification of Clinical-Quality Bacteriophages
Antibiotics are unable to keep pace with infectious diseases, and the use of bacteriophages is a timely solution to that problem. But implementation of bacteriophages involves overcoming some challenges. Broad-spectrum clinical efficacy will require numerous phages. In turn, cost-effective development of purification procedures will require a platform approach in which one basic process can accommodate all species. That includes the ability to reduce endotoxins to appropriately low concentrations despite the high loads that occur in gram-negative production systems. Monolithic chromatography…
Emerging Tools for Exosome Purification and In-Process Monitoring
This eBook introduces new analytical approaches that enable in-line chromatographic detection of exosomes. One approach can discriminate extracellular vesicles from nonvesicle contaminants, and one potentially can discriminate exosomes from other vesicles. Examples illustrate how they enable development of more effective and better documented purification methods. The special qualifications of monolithic chromatography media for exosome purification are discussed. New process tools designed to accommodate some of the special challenges of exosome purification are introduced. Exosomes represent one of several species of…
Intensification of Influenza Virus Purification: From Clarified Harvest to Formulated Product in a Single Shift
Influenza is a global respiratory disease with an estimated mortality of up to a half million people per year (1). The majority of traditional influenza vaccines are still produced in eggs. Downstream processing typically consists of clarification by centrifugation, concentration by ultrafiltration, and purification by ultracentrifugation (2). Recombinant vaccines are most often purified by chromatography. Chromatographic purification of viruses already has achieved major improvements in recovery and scalability (3), but it also is important because it enables virus purification to…