Aggregation is a common cause of protein instability, which renders a biologic product unfit for therapeutic use. Sometimes it is difficult to purify monomeric proteins from oligomers because of similarities in their isoelectric points (pIs). Proteins such as hormones have pI ranges similar to their oligomers and thus can be difficult to separate out using a conventional polishing chromatographic step such as ion exchange. With those pI similarities, removal of oligomers to a considerable extent by ion exchangers can compromise…
Downstream Processing
Removing Oligomers of a Recombinant Human Therapeutic Hormone:
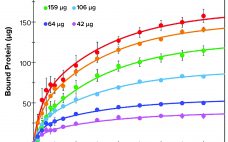
Evaluation of a Novel Peptide-Based Affinity Ligand for Human IgM Purification: Use of an Automated Liquid-Handling System for Rapid Assessment of Binding Kinetics and Capacity
One-step affinity purification of antibodies is a powerful and widely used tool in the biopharmaceutical industry. Although different strategies can be used to purify immunoglobulin isotype G (IgG), the larger antibody isotype IgM has limited options. Human IgM is the largest antibody and primarily exists as a pentamer in the bloodstream (1). IgM molecules exhibit higher avidity than other antibodies do and serve as essential activators for the complement cascade (1–3). Approaches to IgM purification with hydroxyapatite and ion-exchange (IEX)…
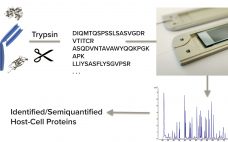
Nontargeted HCP Monitoring in Downstream Process Samples: Combining Micro Pillar Array Columns with Mass Spectrometry
Protein biopharmaceuticals have emerged as important treatments for diseases with otherwise unmet medical needs. These biologics are produced by recombinant mammalian, yeast, or bacterial expression systems. Along with therapeutic proteins, those cells produce endogenous host-cell proteins (HCPs) that can contaminate biopharmaceutical products despite multiple purification steps in downstream processing. Because such process-related impurities can affect product safety and efficacy, they need to be monitored closely. Multicomponent enzyme-lined immunosorbent assays (ELISAs) presently are the workhorse method for HCP testing, with high…
eBook: Ion-Exchange Chromatography for Modern Biopharmaceutical Purification
Understanding the functionalities of chromatography resins can improve the product yield and purity in a biotherapeutic purification workflow. Ion-exchange (IEX) chromatography separates biomolecules on the basis of charge. For several reasons, it is the most widely used separation tool for purification of biopharmaceutical products. IEX is a well-characterized purification method with high binding capacity and flexible selectivity. It also works with mild operating conditions that help to preserve the biological activity of a biopharmaceutical drug substance. That versatility enables several…
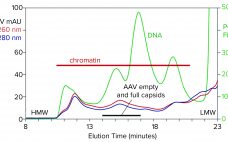
Streamlining Industrial Purification of Adeno-Associated Virus
With its first licensed therapeutic now marketed worldwide (1), adeno-associated virus (AAV) has become a preferred vector for gene therapy. However, unlocking its full potential still poses challenges, many of which are associated with purification. The first involves the transition from upstream to downstream processes. AAV-bearing lysates are laden with debris that foul filtration media and limit or prevent concentration. Another challenge involves reduction of soluble host-cell DNA, which is complicated by its strong association with nucleoproteins. A third involves…
Exploring Resin Modalities for Biotherapeutic Purification
New chromatography supports must demonstrate improved selectivity, and bead technologies must be optimized for high binding capacity and product recovery. Drug manufacturers also need access to expertise and continued support from chromatography suppliers that can assist with method development and design of experiments (DoE) assessments. Working together, these industry groups can accelerate method development, increase process yield, reduce buffer consumption, minimize the number of unit operations, and improve overall process economies. Learn more in this Special Report about how resin…
eBook: Downstream Processing — An Overview of MAb Purification Methods
The downstream harvest, clarification, and purification operations of biologics are essential steps to ensure drug product safety. However, these process steps can be problematic for complex biologics. Compared with processes for traditional small-molecule pharmaceuticals, downstream methods for monoclonal antibodies (MAbs) have higher risks of contamination. Thus, different centrifugation, filtration, chromatography technology and viral clearance/inactivation strategies must be applied to remove dead cells, host-cell proteins, viruses, and other contaminants. Several factors must be considered to determine which methods and technologies are…
Drug Formulations Are Changing:
New Sterile Filtration Challenges in the Changing Landscape of Drug Formulations
Read about the challenges of sterile filtration of high concentration mAbs, liposomes, and lentiviral vectors, and how to solve them in this Special Report. Development of new, complex drug formulations has given us therapeutics with properties that are markedly different from traditional drug types. High viscosity or low surface tension formulations or large viral vector molecules can mean that sterile filtration processes, which are optimized for traditional drug types, are not as efficient for the new, complex formulations. Premature filter…
Development of a Single-Use Hermetic Centrifuge System for Mammalian Harvest with Moderate to High Cell Content
The production of increasingly higher cell densities has stressed the already limited solids-handling capabilities for traditional intermittent ejection centrifuge systems. By contrast, a single-use disc-stack centrifuge based on the solids-flow principle offers distinct advantages for cell culture harvesting. Such benefits include solids handling of high-density cell culture processes and elimination of the separation disruption and aerosol generation associated with the intermittent solids ejection. A single-use system also provides well-established benefits of disposable components — such as removal of steam- and…
A New Runway for Purification of Messenger RNA
A high-performing capture method is a critical bedrock asset for developing industrial purification processes. This is especially true for extended families of products that share highly similar chemical composition. Therapeutic monoclonal IgG is an example. The ability of protein A affinity chromatography to achieve 95% purity in one simple step was the runway that got recombinant immunotherapy off the ground and made it available to millions. In fact, protein A did more. Beyond giving the industry a foundation manufacturing method,…