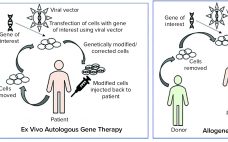
The proposed acquisition of Cognate BioServices will bring services firm Charles River Laboratories autologous and allogeneic cell therapy, plasmid DNA and viral vector manufacturing capabilities. The agreement announced yesterday will see Charles River pay $875 million in cash for the contract development and manufacturing organization (CDMO). Through the deal, Charles River has propelled itself into the manufacturing space, entering a lucrative cell therapy and plasmid production market. âThe addressable market for Cognate’s CDMO services principally cell therapy and plasmid production…
Search Results for: regenerative medicine
Rentschler catapults into the cell and gene therapy space through UK investment
German CDMO Rentschler will set up manufacturing capabilities at the UKâs Cell and Gene Therapy (CGT) Catapult, launching itself into the regenerative medicine space. Family-owned contract development and manufacturing organization (CDMO) Rentschler offers mammalian manufacturing and fill and finish services for biologics but has now expanded into cell and gene therapy production through a deal with the CGT Catapult, based in Stevenage, UK. Rentschler will establish its manufacturing capabilities â including adeno-associated virus (AAV) vector production â through âa significant…
Podcast: Industry veteran moves to Lykan Bioscience, and this time its personal!
In the latest episode of the BioProcess Insider Expression Platform, we explore the evolution of cell and gene therapy production with new COO of Lykan Bioscience, Patrick Lucy. From Repligen, to Lonza, to Pfenex, Patrick Lucy has had an illustrious career. His latest career move is to Lykan Bioscience, a Massachusetts-based contract Manufacturing Services Organization (MSO) and provider of end-to-end solution for cell-based therapies, where he took on the role of chief operating officer (COO) in January. In this podcast,…
Top Trends in Biomanufacturing
Facing an ongoing pandemic, growing pipelines, and a possible capacity crunch, the bioprocess industry is striving to balance its priorities. Those are some of the key issues to watch according to the 17th annual report and survey of biopharmaceutical manufacturing capacity and production from BioPlan Associates. It includes survey responses from 130 decision-makers (from 33 countries) at both bioprocessing organizations and contract manufacturing organizations (CMOs) and responses from 150 bioprocess industry suppliers (1). Top trends from this report are highlighted…
Sanofi: âVaccine heritage puts us in good stead to play in gene therapy spaceâ
Sanofi says it is looking to resolve the âpatchyâ accomplishments in the gene therapy space through a new specialized unit that will leverage technologies from its vaccine division. Sanofi has dabbled in the cell and gene therapy over the past decade but is yet to make its mark. Speaking at the JP Morgan Healthcare Conference this week, CEO Paul Hudson highlighted the importance of the sector going forward but admitted industryâs success in the sector has âbeen patchy.â He told…
Manufacture and Regulation of Cell, Gene, and Tissue Therapies, Part 1: Chemistry, Manufacturing, and Control Challenges
Cell, gene, and tissue (CGT) therapies and other advanced-therapy medicinal products (ATMPs) have made tremendous progress over the past decade. They are different from other biologics and small molecules because of their inherent complexity and variability. Although many unknowns remain about the development of these products, their clinical success has enabled the CGT therapy and ATMP fields to advance rapidly. We are seeing an increase in the number of marketing authorization applications (MAAs) filed in the European Union and new…
Mustang expands âbubble boyâ gene therapy footprint with European CDMO
Mustang Bio has established a European manufacturing base for its lentiviral gene therapy candidate MB-107 through a deal with cell and gene therapy CDMO Minaris. Developed with St. Jude Childrenâs Research Hospital, MB-107 is Mustang Bioâs lentiviral gene therapy for the treatment of X-linked severe combined immunodeficiency (XSCID), also known as bubble boy disease. The candidate, which received US FDA Orphan Drug Designation in September, is manufactured from a facility in Worcester, Massachusetts but the firm is now looking outside…
CDMO Minaris invests $64.5m to triple capacity in Europe
The cell and gene therapy CDMO will build additional facilities at its German and Japanese locations. The contract development and manufacturing organization (CDMO) specializing in cell and gene therapies announced two separate investments of $40.7 million (â¬34.2 million) into its Ottobrunn, Germany, site, and $23.8 million for its Yokohama, Japan, site. Both regions now come under the umbrella of Minaris Regenerative Medicine, a subsidiary of Showa Denko Materials, after a rebrand in September to unify the name of its three…
Bluebird: CMC and COVID delays push sickle cell gene therapy BLA back to 2022
Bluebird bio has asked the US FDA to take a flexible and innovative approach to the CMC comparability data review for gene therapy candidate LentiGlobin. It had been estimated that bluebird bio was aiming to submit a Biologics License Application (BLA) for its gene therapy candidate LentiGlobin (autologous CD34+ cells encoding βA-T87Q-globin gene) in the second half of 2021. But following US Food and Drug Administration (FDA) concerns, guidance has been put back by a year, management said on a…
With $30m in hand, Ori looks to advance closed cell therapy tech
Ori Biotech will use $30 million from a Series A financing round to finalize and test its closed and automated cell and gene therapy technology. In January, Ori Biotech came out of stealth mode through a $9.4 million seed round, introducing the cell and gene therapy space to its technology platform, which aims to significantly reduce the cost of production of such therapies. Nine months on and the firm has raised a further $30 million in a Series A financing…