This webcast features:Â Dr. Peter Kilbride and Dr. Julie Meneghel, Senior Research Scientist and Cryobiologist, GE Healthcare Life Science In this webinar, we will address fundamentals of cryobiology to (1) understand why a cryopreservation protocol is needed, (2) see what happens biologically to cells while cooling, and (3) allow attendees to design an optimized protocol for their cell type and cryopreservation configuration (e.g., sample size). We will cover the importance of applying controlled-rate cooling and how to best tune it to…
Search Results for: regenerative medicine
Vaccines, plasma and stem cells: How industry hopes to take on COVID-19
The biopharma space has stepped up its efforts to both prevent and treat the coronavirus (SARS-CoV-2) that is threatening to bring the world to its knees. A month is a very long time when it comes to infectious diseases. The first cases and deaths from the novel coronavirus (COVID-19) led to a response to contain the virus, but the difficulties of containment and the nature of international travel means cases and deaths have become global. The latest statistics place the…
Better Bioprinting Ahead: Breakthroughs and Remaining Challenges
Bioprinted organs soon could revolutionize clinical trials, transplantation, and regenerative medicine. But as Chris Lo reminds us in a new GlobalData report (1), several technical hurdles must be negotiated before biopharmaceutical companies can harness three-dimensional (3D) bioprinting for such purposes. BPI explores persistent printing problems and promising solutions below by analyzing Lo’s report alongside commentary from founding editorial advisory board member Bill Whitford (bioprocess strategic solutions leader at GE Healthcare Life Sciences), Lev Gerlovin (vice president in the life sciences…
Bioassay Evolution: Finding Best Practices for Biopharmaceutical Quality Systems
Bioassays help drug developers determine the biological activity (potency) of their products, which has been a biopharmaceutical critical quality attribute (CQA) since long before that concept had a name. Because of their complex nature, bioassays are among of the most challenging experiments to perform reliably with dependably accurate results. Consistent assay performance requires a controlled environment and qualified reagents; skilled analysts who understand cell physiology, regulatory requirements, and the latest techniques; and assay protocols that are intelligently developed, characterized, and…
Akron says biopharma needs GMP framework for ancillary materials
Akron Biotechnology says biopharma needs a common GMP framework for ancillary materials used in the production of advanced therapies Ancillary materials (AMs) are components and reagents used during cell therapy production. They are not supposed to be present in the finished products, but often are which can impact quality and safety. Despite this, at present there are no specific regulations governing the composition, compliance, and qualification of AMs. Instead there are several independent GMP frameworks for such materials. These include…
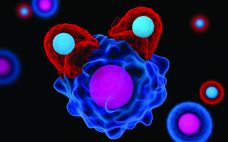
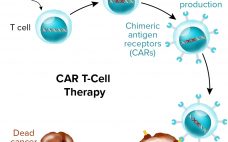
Challenges and Opportunities in CAR T-Cell Development and Manufacturing
Just about anyone in the biopharmaceutical industry will tell you that cost is now the primary concern in cell and gene therapy development. It hasn’t even been a decade since “manufacturability” was the main issue at hand — and cost has risen organically from related discussions. Regenerative medicine evolved from medical research rather than from drug-development companies, and technologies that worked in clinical settings haven’t translated directly to manufacturing facilities. Cost is often the problem. Early product successes (that ultimately…
Cell Banking for Cell and Gene Therapy: Regulatory, Ethical, and Scientific Considerations
Regenerative medicine holds great potential for human disease management, with hundreds of cell and gene therapy (CGT) products for tissue/organ reconstitution or replacement in different stages of development and clinical testing for toxicity, safety, and efficacy. For example, currently more than 60 CGTs have marketing authorization (although many with only conditional approval) from central regulatory agencies worldwide (1). Those products are treating conditions such as hematopoietic malignancies, immunological disorders, and cartilage disorders. Most of those treatments use culture-expanded autologous or…
Do We Need Separate Regulations for Advanced Therapies?
Before we had the 21 CFR 1271 regulation for tissue therapies, the US Food and Drug Administration (FDA) had determined that regenerative medicine was exceptional enough to warrant its own regulations for good manufacturing practice (GMP). Since 2001, the tissue industry has adapted to those new rules while the FDA stepped up enforcement over time. When a cell or tissue product is regulated under 21 CFR 1271, its specific regulations apply before the general regulations for biologics and drugs. But…
CAR-T at the Crossroads: Is Allogeneic the Way to Go?
As cell therapies move through the clinic toward commercialization, respondents to an Informa Connect industry survey are beginning to look to allogeneic — or off-the-shelf — products as “the next big thing.” Almost 200 people contributed to the Cell Therapy Analytics Report, revealing their current positions within the burgeoning cell and gene therapy space and offering their thoughts and predictions for the future. Most survey respondents work within companies developing oncology products. Of those, the largest group (41%) said that…
Top 10 advanced therapy milestones of 2019: Patient access takes center stage
CRISPR, capacity, and consolidation powered the cell and gene therapy space in 2019, but a proactive focus on patient access topped Falcon Therapeutics CEO Susan Nichols’ annual roundup. In what has become one of the most anticipated presentations at the Phacilitate conference, Susan Nichols, CEO of cell therapy firm Falcon Therapeutics, laid out the top 10 events of the previous year that have shaped the regenerative medicine space, driving conversation, investment, and innovation. The top spot in 2019 focused on…