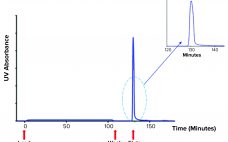
Novel therapeutics based on extracellular vesicles (EVs) recently passed a critical development milestone. During 2020, some of the first experimental EV products developed by biopharmaceutical companies entered human clinical trials (1–3). EVs are nanometer-sized, lipid-wrapped spheres released by almost every cell type in the human body. EVs are loaded with a cargo of proteins, lipids, and RNA, and they are tagged with surface markers that favor uptake by target cells. Thus, EVs are a key mode of cell-to-cell communication (4).…
Search Results for: regenerative medicine
COVID-19 driven CDMO investments to benefit wider sector
COVID-19 driven investments in vaccine production capacity will help relieve existing bottlenecks as the pandemic recedes, according to the authors of a new report. Demand for vaccine production capacity increased significantly last year as pharmaceutical industry efforts to develop effective jabs accelerated. The contract development and manufacturing organization (CDMO) sector’s response has been to invest in vaccine and vector production capacity, says Fiona Barry from GlobalData. “We’re seeing very high investment in vaccine-related contract manufacturing, by dedicated CDMOs and by…
Novartis to close Swiss stability testing site by 2023
A move to specialized and personalized medicines has led Novartis to plan closure of a Swiss stability testing facility by 2023. Big Pharma has shifted its pipelines away from traditional small molecule drugs to biologics and regenerative medicine over the past 20 years. As such changing needs at Novartis has led the firm to announce it is closing its stability testing facility in Locarno, Switzerland by 2023. “Novartis plans to adapt its product testing capacity in Locarno, Switzerland, to the…
The Unique Benefits of Presterilized Fluoropolymer Bottles: Case Studies in Vaccine Delivery Applications
This webcast features: Eric Isberg, Vice President, Life Sciences, Savillex Fluoropolymers are the ultimate materials for use in bioprocess applications, providing increased purity, compatibility, inertness, and security for process fluids and bulk product. Bottles and vials manufactured from fluoropolymers have the added benefits of having virtually no leachables and extractables, a very wide service temperature range including performance down to liquid nitrogen temperatures, and unmatched durability, including being essentially unbreakable during use. This presentation will highlight the benefits of presterilized…
Untapped Potential of Tissue Engineering: The Three Obstacles Holding It Back
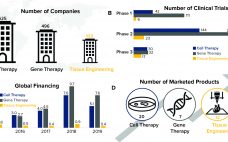
Regenerative medicine is the interdisciplinary field comprising tissue engineering, cell therapy, and gene therapy. These biopharmaceutical modalities, also referred to as advanced therapies, are growing rapidly, characterized by groundbreaking therapeutic advances that have the potential to change how healthcare providers deliver care. As Figure 1 shows, cell and gene therapies have gained traction over the past decade, as evidenced by large increases in investment and the number of marketed products. By contrast, tissue engineering investment and product commercialization has lagged…
Manufacture and Regulation of Cell, Gene, and Tissue Therapies: Part 2 — Regulatory Guidances
The US Food and Drug Administration (FDA), the European Medicines Agency (EMA), the Medicines and Healthcare Products Regulatory Agency (MHRA), and Japan’s Pharmaceutical and Medical Devices Agency (PMDA) all offer support and guidance for developers of advanced therapy medicinal products (ATMPs). Some agencies have issued guidelines to help companies through different stages of product development — from research and development to marketing authorization and postauthorization activities. Such guidelines are updated regularly as more knowledge becomes available from the development and…
Tenaya talks advantages of inhouse capabilities for CGT firms
Having internal AAV capabilities gives Tenaya Therapeutics control over its processes, timelines, and costs, but CEO Faraz Ali says it will also likely use third-party manufacturers as pipelines progress. Fresh from a $106 million Series C funding, South San Francisco-based cell and gene therapy firm Tenaya Therapeutics is establishing a dedicated facility for AAV (adeno-associated virus) manufacturing to support its emerging portfolio of gene therapy products. “We have leased a 94,000 square-foot space for our new cGMP facility, and we…
eBook: Buffers — Navigating New Demands on Downstream Raw Materials
Bioreactor titers for monoclonal antibody (MAb) processes have increased significantly since the dawn of the biopharmaceutical industry, yet such gains have instigated bottlenecks for critical high-volume raw materials used in downstream processing, such as buffer solutions. As downstream purification is required for most, if not all, biopharmaceutical products, buffers and their preparation are topics that concern nearly every drug company. But those topics rarely receive direct attention. This BPI eBook explores what factors prompted the current buffer bottleneck and what…
Stirling Ultracold fifth acquisition in 2 years for BioLife
Supplier of services and bioproduction tools BioLife Solutions has merged with developer and manufacturer of ultra-low temperature freezers Stirling Ultracold. “We continue to execute on our M&A strategy with another acquisition that superbly complements our product portfolio. Combining Stirling’s -20°C to -86°C freezer systems with our liquid nitrogen products provides complete ultra-low temperature cold-chain infrastructure critical for personalized medicine,” said BioLife’s CEO, Mike Rice. The transaction is expected to close during the early second quarter of 2021 with BioLife planning…
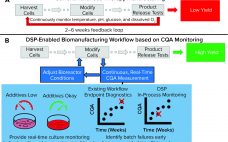
Improving Cell Manufacturing Outcomes Using In-Line Biomarker Monitoring
Cell-based advanced therapies are changing modern medicine dramatically. Immunotherapies such as chimeric antigen receptor (CAR) T-cell therapies are treating different forms of cancer. Gene therapies are reversing the course of inherited diseases, and tissue-engineered medical products are restoring, maintaining, and replacing damaged organs (1–4). The development of new advanced therapies is booming. As of January 2020, the US Food and Drug Administration (FDA) has reported more than 900 investigational new drug (IND) applications for cell and gene therapy products. However,…