Manufacturing issues and a scarcity of new commercial products leave predictions that 10-20 cell and gene therapy approvals each year by 2025 somewhat fanciful, says Dark Horse Consulting. In his plenary address at the Phacilitate conference yesterday, Anthony Davies, founder of cell and gene therapy specialist firm Dark Horse Consulting, reflected on the difficulties the sector has faced since the high of 2017 when three products achieved US Food and Drug Administration (FDA) approval: Kymriah (tisagenlecleucel) and Yescarta (axicabtagene ciloleucel),…
Search Results for: regenerative medicine
The 12 trends of Christmas: A look back at 2019
CAR-T partnerships, Biopharma megamergers, biotech funding, and an eye on China. Bioprocess Insider presents the biggest stories of the year⌠through song! Tâwas the days before Christmas, And all through the dept. Not a story was breaking and journalists wept. So why not look back at the news of the year, The editor suggested over a beer⌠In the past twelve months Bioprocess Insider brought to you (To the tune of traditional Christmas song âThe Twelve Days of Christmasâ): A…
The Proof Is in the Data: Extractables and Leachables
Extractables and leachables (E&Ls) must be addressed in material and process validation programs. Extractables are compounds that can be extracted from a material in the presence of solvents with varying polarity under extreme conditions. Materials manufacturers should make extractables guides available to end users. Leachables are compounds that migrate from a material in the presence of an actual formulation under normal process operating conditions. Extractables information can be helpful as a basis for evaluation of potential process-equipmentârelated leachables (PERLs)testing. However,…
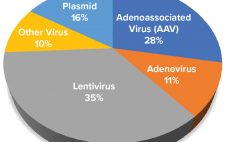
Capacity Analysis for Viral Vector Manufacturing: Is There Enough?
Advanced therapy medicinal products (ATMPs) are engineered to replace defective, disease-causing genes to compensate directly for a genetic defect or to encode a therapeutic protein construct (e.g., chimeric antigen receptor, CAR) for disease treatment. In most instances, a viral vector delivers the engineered genetic payload, targeting cells in situ or ex vivo through cellular modification, expansion, and infusion into a patient. Clinical successes of ATMPs bolstered by regulatory approval of products such as Luxturna (voretigene neparvovec-rzyl, Spark Therapeutics), Kymriah (tisagenlecleucel,…
Measure Twice, Treat Once: Navigating the Regulatory Landscape of Assay Development to Ensure High-Quality CGT Products
Cell and gene therapies (CGTs) are a novel and fast-growing class of transformative therapies designed to address gaps in traditional treatment strategies of some of the most severe diseases. By definition, gene therapy âseeks to modify or manipulate expression of a gene to alter the biological properties of living cells for therapeutic useâ (1). That can be either an in vivo delivery of a gene or delivery of a gene to a patientâs cells that are manipulated outside of the…
Thermogenesis cell preservation tech to drive CAR-T cancer trial eligibility
Rebranded Thermogenesis used its quarterly call to outline plan to target unmet tech and manufacturing needs in the CAR-T sector. The firm â known until November 1 as Cesca Therapeutics â will pitch its cell processing tools and its new services joint venture â Immunecyte â as a way to help reduce CAR-T manufacturing, storage and distribution costs. CEO Chris Xu told analysts on Thermogenesisâ third quarter call that Immunecyte â formed with Healthbanks Biotech last month â would be…
Biolife buys CBS, turns focus on integrating cold-chain acquisitions
Biolife Solutions says cold storage tech firm Custom Biogenic Systems (CBS) is likely to be its last acquisition for a while as integration will be the focus in 2020. The US cell and gene therapy tool firm announced the deal this week, citing CBSâ portfolio of liquid nitrogen lab freezers and cryogenic equipment as well as its design and manufacturing capabilities as motivations. CBS CEO, founder, and sole shareholder John Brothers received $11 million (âŹ10 million) in cash and $4…
Abuse in cell banking services a global problem, says industry consortium
The International Society for Cell and Gene Therapy (ISCT) has formed a consortium to tackle what it says is a rising number of unscrupulous and unproven cell banking players. With the rise of interest in the cell and gene therapy sector, industry and the market have been plagued with unproven products and services from rogue actors looking to profit from ill-informed and sometimes desperate patients. The US Food and Drug Administration (FDA) laid down a framework to tackle unapproved stem…
Cognate buys Cobra to boost gene therapy CDMO capabilities
Cell therapy CDMO Cognate Bioservices will add plasmid DNA and viral vector capabilities through the acquisition of Swedish manufacturer Cobra Biologics. Memphis, Tennessee-based contract development and manufacturing organization (CDMO) Cognate has entered into an agreement to acquire Cobra for an undisclosed fee, led by existing Cognate investor EW Healthcare Partners. The deal adds to Cognateâs presence in the regenerative medicine space by bringing on board plasmid DNA and viral vector manufacturing capacity and expertise, complementing its own autologous and allogeneic…
eBook: Bioassays for Biopharmaceuticals: Finding Best Practices in a Quality Systems World
Bioassays are complex and challenging experiments to run reliably with accurate and dependable results. Consistent performance requires a controlled environment and qualified reagents; skilled analysts who understand cell physiology, regulatory requirements, and the latest techniques; and assay protocols that are intelligently developed, characterized, and validated. Here, BPIâs senior technical editor discusses bioassay best practices with representatives of the Biopharmaceutical Emerging Best Practices Association (BEBPA) organization. Topics span quality by design, assay validation, cell banking, potency testing and host-cell protein monitoring,…