There‚Äôs a funny meme going round social media about the naivete of conspiracy theorists: ‚ÄúMy gut is that most of them have never been project managers. Their optimism is adorable.‚ÄĚ And the adage may be that ‚Äútoo many cooks spoil the broth,‚ÄĚ but I think it‚Äôs more like ‚Äútoo many stakeholders can overcomplicate anything.‚ÄĚ That can apply to small teams working on relatively simple projects (a group of editors working on a single BPI issue, for example) as much as…
Search Results for: regenerative medicine
Hurdles Ahead for Cell and Gene Therapy Makers
Significant growth of the cell and gene therapy (CGT) pipeline in recent years demonstrates the enormous potential of these modalities to treat or even cure otherwise intractable diseases. Several CGT products have been approved for clinical use over the past five years. More than 75 such products have come to the market around the world so far. They include chimeric antigen receptor (CAR) T-cell therapies that involve genetic engineering of patient cells ex¬†vivo as well as in vivo gene therapies…
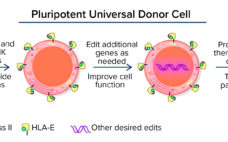
Novel Technologies for Advancing Allogeneic Cell Therapies
In many ways, cells are the ultimate therapeutic product. They can integrate and participate in different biologic processes and replace missing biological functions. Cell therapies are dynamic, versatile, and with the appropriate engineering, capable of influencing and correcting disease processes robustly. Cell therapies essentially are living medicines, and their adaptability contrasts with conventional drug modalities that generally have only a single specific target or effect. Because cell therapies are highly complex modalities, their scientific and R&D challenges are different from…
Advancing Logistics for ATMP Manufacturing
Advanced therapy medicinal products (ATMPs) hold much potential for improving healthcare. They offer hope for treating or even curing patients. The biopharmaceutical industry has recognized the importance of making such therapies accessible to as many people as possible. To provide personalized ATMPs, biomanufacturers are shifting toward flexible, patient-centered production processes. A Paradigm Shift in ATMP Manufacturing Ex vivo cell and gene therapies are particularly promising approaches to personalized regenerative medicine. Thus, it is no surprise that the numbers of US…
Aseptic closed small-volume processing: Today’s options
Small-volume biopharmaceutical processes continue to grow as more biopharmaceutical, cell therapy and gene therapy companies develop products. A 2021 Association for Regenerative Medicine (ARM) report states that there are 1,085 cell, gene and tissue-based therapy developers worldwide. These companies are engaged in many small-volume (<10L) processes including early-stage drug development and autologous therapies. The increase in small-volume processes coincides with the ongoing need to create fast, reliable aseptic closed systems. Historically, few convenient options have existed to facilitate sterile processing.…
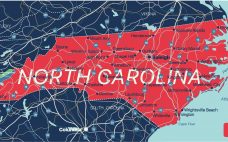
Carsgen’s US CAR-T plant open for business
Carsgen Therapeutics‚Äô new facility in North Carolina is poised to begin making candidate CAR-T cell therapies for trials after passed an inspection. The facility in Research Triangle Park (RTP) ‚Äď which official opened this week – was issued with a certificate of compliance after being visited from inspectors from Durham city council. Company president Richard Daly welcomed the decision as an important expansion of Carsgen‚Äôs manufacturing network. ‚ÄúRTP manufacturing facility plus two Carsgen existing GMP facilities in¬†Shanghai, China¬†will enhance our…
EUAs for rare disease advanced therapies? Not likely, says FDA
The FDA has dismissed using special emergency authorization powers for rare disease advanced therapeutics but does hope to significantly speed up its feedback to sponsors. The US Food and Drug Administration‚Äôs (FDA) Emergency Use Authorization (EUA) authority allows the use of unapproved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions caused by chemical, biological, radiological, and nuclear (CBRN) threats when certain criteria are met. Most recently, EUA powers have…
Univercells and RoosterBio target regen med commercialization
The partnership aims to enhance manufacturing of extracellular vesicles (EVs) using continuous bioprocessing technologies to make regenerative medicine cheaper. The aim of the collaboration is to deliver exosome manufacture, using Univercells‚Äô scale-X hydro fixed-bed bioreactor and RoosterBio‚Äôs human mesenchymal stem/stromal cells (hMSC) bioprocess and engineering media systems to establish EV produ¬†ction. ‚ÄúA first step will be to evaluate the performance of the EV manufacturing process in the scale-X bioreactor and benchmarking it against a reference process (microcarriers process in the…
Excellos: ‚ÄėThere is no better time to enter cell therapy CDMO space‚Äô
With the¬†San Diego Blood Bank at its foundation and $15 million of funding in hand,¬†Excellos¬†launches itself in the cell therapy¬†contract¬†manufacturing¬†sector.¬† A new contract development and manufacturing organization (CDMO) has arisen in San Diego, California this week: Excellos Incorporated, which officially launched on the back of $15 million in growth funding from Telegraph Hill Partners (THP).¬† The new entity ‚ÄĒ announced during¬†Phacilitate‚Äôs¬†Advanced Therapies Week¬†conference in Miami, Florida ‚ÄĒ¬†already has an operational cGMP manufacturing facility for Advanced Therapies but according to CEO…
Limula on the future of cell therapies: Closed, automated, decentralized
An in-situ centrifugation-based point-of-care system could help push industry towards the decentralized and automated model needed to make cell therapies affordable and reliable, according to Limula Biotech. The approval of Novartis‚Äô Kymriah (tisagenlecleucel) in 2017 was a milestone in the advanced therapy space, marking the arrival of the first genetically-modified autologous T-cell immunotherapy to the market. Since then, a handful of other autologous cell therapies have received the regulatory thumbs up ‚Äď most recently Bristol-Meyer Squibb‚Äôs Breyanzi (liso-cel) and Abecma…