Since 2018, global bioprocessing capacity has grown from 16.5 million liters (1) to 17.4 million liters. Although output has continued to expand at around 12% overall, that rate represents a significant slowing in capacity growth as the industry moves toward greater productivity and efficiency. Trends that we have tracked in the BioPlan Associates annual report of biopharmaceutical manufacturing capacity and production (2) for over 17 years correlate with that finding. Titers are increasing; single-use technologies have reduced the need for…
Author Archives: Ronald A. Rader
Top Trends in Biomanufacturing
Facing an ongoing pandemic, growing pipelines, and a possible capacity crunch, the bioprocess industry is striving to balance its priorities. Those are some of the key issues to watch according to the 17th annual report and survey of biopharmaceutical manufacturing capacity and production from BioPlan Associates. It includes survey responses from 130 decision-makers (from 33 countries) at both bioprocessing organizations and contract manufacturing organizations (CMOs) and responses from 150 bioprocess industry suppliers (1). Top trends from this report are highlighted…
Worldwide Biopharmaceutical Manufacturing Capacity Analysis: Growth Continues Across the Board
While the growth in biopharmaceutical manufacturing capacity in developed, major market countries is continuing its slow and steady climb, developing regions often are seeing double that growth rate. Over the past eight years, as detailed in the “About the Data” box, our company’s index of the top 1,000 biomanufacturing facilities (1) has tracked and ranked bioprocessing facilities worldwide in terms of known or estimated bioprocessing capacity (cumulative onsite bioreactor volume) number of biological products manufactured at clinical scale commercial scale…
eBook: The Commercial Expression Systems Market — What Has Changed in the Past Decade
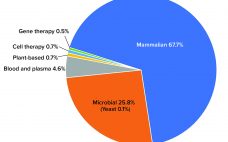
A decade ago, BioPlan Associates prepared the findings of its 2008 directory of expression system technologies that were being promoted or considered likely to be suitable for commercial licensing for biopharmaceutical manufacturing (1). Due in part to the relatively slow advances in this critical area of bioprocessing, this study remains perhaps the only directory of biopharmaceutical-relevant expression systems available for licensing. Here I discuss aspects of related bioprocessing technologies that have and have not changed in the past decade. Expression…
Biosimilar Markets and Regulation: Which Countries Are Going All In?
The pipeline of follow-on (biosimilar and biobetter) products in development for the US, EU, and other major markets is very healthy. It includes nearly 800 biosimilars, about three-quarters of which are presumed to be targeted for major markets, and about 500 biobetters in development. Nearly 1,200 follow-on biopharmaceutical products in the development pipeline are intended to compete with more than 100 currently marketed biopharmaceuticals. This is not just an opportunity in the Western world; biosimilars development is expanding globally. But…