This eBook gauges shifting expectations for the gene therapy industry amid the COVID-related uncertainties and clinical setbacks of the past couple years. BioProcess Insider founding editor Dan Stanton reports on the January 2022 Phacilitate Advanced Therapies Week event, specifically a standing presentation on the 10 most important industry drivers from the past year. Since 2017, advancements in gene therapies have featured prominently in these presentations. In 2021, gene therapies again made the list, but this time for more troubling reasons,…
Cell/Gene Therapies
eBook: Gene Therapies —
Realization of Quality By Design and Beyond: The Intelligent Cell Processing System
Cells are used as raw materials, intermediates, and final products in biopharmaceutical and cell therapy manufacturing. Because living cells are always in a dynamic state, their characteristics must be kept within specified ranges throughout bioprocessing to preserve their utility. Cell population expansion without changing the original cell properties is key for obtaining the required number of cells at the next step. However, limited process data are collected during the expansion phase — information that could be used to understand and…
Get High-Throughput, Definitive Identification of Viable Cells and More in Your Cell Therapy
Analyzing for viable cells using traditional methods such as flow cytometry often encounters clogging resulting in the loss of precious cell therapy samples. Not only is it low throughput, but incredibly complicated, which can lead researchers to misidentify cellular and non-cellular material and confuse cell viability results with product-purity issues. Additionally, it is a regulatory requirement for all injectable drug products be characterized for sub visible particles (SVP) and aggregates that may form during a manufacturing process. With Backgrounded Membrane…
Addressing Critical Issues in Cell and Gene Therapy Manufacturing
Recombinant lentivirus (LV) and adeno-associated virus (AAV) are critical components of cell and gene therapies, which show great promise for treatment of diseases from genetic disorders to cancer. Accordingly, there is an unprecedented need for high titer and large-scale viral vector manufacturing processes to support the growing number of researchers developing biotherapeutics for immunotherapy. Download this Special Report, highlighting: Evolutions of gene therapy manufacturing Improvements to GMP raw-material sourcing. Role of transfection reagents like VirusGEN® GMP as a turnkey element…
Reducing Cell and Gene Therapy Development Time and Cost with New Purification Strategies
The past 40 years have ushered in the most advanced medicines the world has ever seen, with tremendous improvements in biomanufacturing technologies to enable their development. Advances in production technology have brought significant improvements in upstream productivity, which then caused bottlenecks in downstream processing. Although many bottlenecks have been resolved for most biologics, new modalities such as gene therapies and mRNA vaccines are driving the need for differentiated purification solutions. Meanwhile, pressures to increase efficiency and reduce costs continue to…
eBook: Cell and Gene Therapies — Optimization Strategies for Processing and Potency
Although relatively new to the biopharmaceutical industry, cell and gene therapy development and manufacturing are advancing rapidly. At Informa Connect’s September 2021 Cell & Gene Manufacturing & Commercialization Conference and Exhibition, held in Boston and online, presentations reviewed concerns that arise when processing complex therapies and highlighted some innovative strategies for surmounting those obstacles. Most of those approaches described during the event used data-driven solutions, with each step building on the information gained from the previous one. High-throughput technology platforms…
Optimizing the AAV Transfection Process in Suspension Cells
Given the potentially curative nature of gene and gene-modified cell therapies and the successful launches of several such products over the past five years, markets and investments are growing significantly in the sector. In the United States alone, the US Food and Drug Administration has granted approval for several genetic therapies, including Strimvelis (autologous CD34+, developed by GlaxoSmithKline and later sold to Orchard Therapeutics) in 2016 Luxturna (voretigene neparvovec, Spark Therapeutics), Yescarta (axicabtagene ciloleucel, Kite Pharma/Gilead), and Kymriah (tisagenlecleucel, Novartis)…
Viral Safety of Viral Vectors:
Special Concerns Arise When the Virus Is the Product
As anyone who has focused on host-cell proteins as process contaminants can tell you, trying to purify a specific type of molecule from a large mixture of many similar molecules is like trying to find a few particular needles in a huge pile of varied needles. The same could be said for purifying viral vectors from cell culture fluids. When viruses are the products, unwanted viruses are contaminants that must be separated away — or better yet, prevented from being…
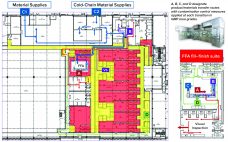
Formulation, Fill and Finish of Lentiviral Vectors: Part 1 — Case Study in Facility and Process Design
Over the past few years, Oxford Biomedica Ltd. (OXB) has developed and implemented a fill–finish platform (“Oxbox,” Figure 1) at its viral vector processing facility in the United Kingdom. The facility includes four segregated bulk viral-vector drug substance (VS) suites, where closed systems and bioburden control processes apply, and two viral-vector drug product (VP) fill–finish suites that apply aseptic processing, with space for expansion by scale-out as product output demand increases. Segregated suites enable the facility to process different viral…
Spurring on Innovation in Gene Therapy Development
Gene therapies based on adenoassociated virus (AAV) vectors hold promise for treating myriad conditions. Immunogenicity remains a challenge for such products, however. With support from PerkinElmer, Roland W. Herzog (professor of pediatrics and Riley Children’s Foundation professor of immunology at the Indiana University School of Medicine) joined Nagendra Venkata Chemuturi (scientific director of global research for drug metabolism and pharmacokinetics, DMPK, at Takeda Pharmaceuticals) to deliver a BPI “Ask the Expert” presentation exploring strategies for minimizing immune responses to AAV-based…