Biopharmaceutical companies are racing to develop vaccines that mitigate the COVID-19 pandemic, taking a wide range of vaccine-development approaches that include traditional modalities and cutting-edge technologies based on DNA and RNA. Vaccine developers are leveraging robust manufacturing concepts and integrated processes to shorten timelines. Advanced analytics also are playing a critical role in ensuring the safety and efficacy of those emerging vaccines. A New Wave of Vaccines Vaccines based on attenuated viruses entail development timelines ranging from four years to…
Analytical
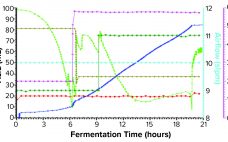
Oxygen Control Strategy and Yield of Recombinant Antibody Fragments Produced in Fermentation
Immunoglobulin molecules are used extensively in therapeutic treatments, diagnostic applications, and fundamental academic research. Traditionally, full-length antibodies and smaller fragments such as the recombinant antigen-binding fragment (rFab) are produced through mammalian cell culture. rFabs also are small enough to be produced in Escherichia coli through fermentation (1, 2). Because disulfide bonds cannot be formed efficiently in the reducing cytoplasm of E. coli, rFabs are supplemented most commonly with a signal sequence that directs them to the more oxidizing bacterial periplasm…
Bioreactor Automation Driven by Real-Time Sensing: Enhancing Productivity Through Accurate, Efficient Glucose Control
In the quest for improved quality and productivity in drug manufacturing, the industry is moving toward increasing use of bioreactor systems with real-time integrated monitoring and advanced analytics that can enable automation, drive performance, and improve data-rich quality control. However, there are multiple options for sensors and technologies that monitor important cell-culture variables or critical process parameters (CPPs). Furthermore, cell culture vessels can be disposable single-use bioreactors (SUB) or reusable glass or stainless-steel models. They can operate in stirred tanks,…
A Universal Assay Determination Method for Antisense Oligonucleotides: A New Slope Spectroscopy Method
Antisense oligonucleotides (ASOs) are short, synthetic, single-stranded oligodeoxynucleotides that can alter RNA and reduce, restore, or modify protein expression through several distinct mechanisms. ASO technology has become an important drug discovery platform for most major pharmaceutical companies. To date, six antisense drugs have been approved by regulatory agencies to treat diseases spanning viral infections, hyperlipidemias, and neurological diseases. More than 50 additional ASO drugs are in clinical trials. For an ASO drug product, an assay of its active pharmaceutical ingredient…
eBook: Biomarkers — Improving Clinical Studies to Enhance Commercial Success for Biologics
The biopharmaceutical industry continues to invest heavily in technologies for identification of predictive biomarkers. Drug developers want not only to find quantitative evidence that their therapies will work as designed, but also to anticipate which patient populations will respond positively to those regimens. Doing that could streamline clinical trials, accelerate approvals, and ultimately improve patient outcomes. Advances in next-generation sequencing and increases in computational capability now are facilitating biomarker inquiries, especially in the realm of immunooncology. However, predictive biomarkers remain…
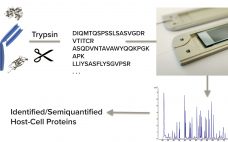
Nontargeted HCP Monitoring in Downstream Process Samples: Combining Micro Pillar Array Columns with Mass Spectrometry
Protein biopharmaceuticals have emerged as important treatments for diseases with otherwise unmet medical needs. These biologics are produced by recombinant mammalian, yeast, or bacterial expression systems. Along with therapeutic proteins, those cells produce endogenous host-cell proteins (HCPs) that can contaminate biopharmaceutical products despite multiple purification steps in downstream processing. Because such process-related impurities can affect product safety and efficacy, they need to be monitored closely. Multicomponent enzyme-lined immunosorbent assays (ELISAs) presently are the workhorse method for HCP testing, with high…
Making Safe and Effective CAR T Cells: How Droplet Digital PCR Can Improve Their Quality Control
Chimeric antigen receptor (CAR) T cells first entered US clinics in 2017 (1), and this therapeutic modality holds tremendous potential as one of the most effective forms of personalized cancer care ever to reach patients. The revolutionary impact of CAR T-cell therapy comes from its ability to rewire our own immune defenses to kill cancer cells: It essentially modifies a patient’s naturally existing immune cells to boost their recognition and attack of cancer cells so that the person’s own immune system…
Practical Considerations for Statistical Analyses in Continued Process Verification
Several statistical techniques can be used to assist in monitoring biopharmaceutical product quality attributes as part of continued process verification (CPV) activities. These include run charts, control charts, and capability analyses. Below, I provide an overview and recommendations on statistical strategies when developing a CPV program, considering the expected behavior of manufacturing results in the biopharmaceutical industry. Presence of Autocorrelated Data In a previous study, I highlighted the tendency for data to be positively autocorrelated (values are closely related to…
Analytical Support for Biologics: A Conversation with Almac Sciences
Almac Sciences (a member of the Almac Group) recently expanded its suite of analytical solutions to include biologics testing. This follows a 2019 expansion of the company’s facility in Athlone, Ireland, where it provides a comprehensive range of flexible pharmaceutical testing services to support drug development programs adhering to industry regulations and good manufacturing practice (GMP) standards. “Biologics have gained huge traction in the past decade and are poised for stronger growth in the coming years with potential to significantly…
Ultrasonic Flow and Bubble Sensors: Optimize Process Quality in Single-Use Bioprocessing Applications
Process monitoring in laboratory and production environments enables continuous control and optimization of critical process parameters. Hence, the early detection of errors is an effective means of increasing process efficiency and reproducibility, improving quality and safety parameters, and reducing long-term costs. Highly precise and flexible noncontact clamp-on flow and bubble sensors are useful instruments to fulfill these goals. They can be applied effectively to buffer and media preparation, chromatography and filtration systems, bioreactors and fermentors, feed and transfer lines, and…