Major biopharmaceutical companies are teaming up with academics and the Bill & Melinda Gates Foundation to develop new biomanufacturing cell lines and methods. The project — known as the AltHost Consortium — is exploring innovative ways to produce biologics and vaccines for clinical usage in diseases from diabetes to cancer. Lead researcher J. Christopher Love at the Massachusetts Institute of Technology (MIT) likens this precompetitive, open-access collaboration to the early days of the biopharmaceutical industry. “When biomanufacturing first emerged as…
Upstream Processing
Cell-Free Expression: A Technology with Truly Disruptive Potential
Bioprocess engineer Beatrice Melinek is a postdoctoral research fellow at University College London’s Future Targeted Healthcare Manufacturing (FTHM) Hub, where she focuses on the use of cell-free protein synthesis (CFPS) as a platform for distributed production of stratified biotherapeutics. Previously Melinek specialized in purification of viral vectors and vaccines, with an engineering doctorate (EngD) in biochemical engineering and postdoctoral experience in UCL’s hematology department developing a new chromatography-based analytical method for measuring empty and full adenoassociated virus (AAV) capsids. She…
Technologies and Innovations: A Discussion with Selexis SA
Pierre-Alain Girod is chief scientific officer (CSO) for Selexis SA. He holds a PhD in plant biochemistry from the University of Lausanne in Switzerland and completed a postdoctoral fellowship at the University of Wisconsin in Madison, WI, on the degradation of proteins by the ubiquitin pathway. Girod returned to Switzerland in 1993, where he discovered a family of sequences that are involved in the epigenetic regulation of genes. That discovery subsequently has been used to express therapeutic proteins in the…
Rapid Development of Viral Vector Production Processes: Iterative Parameter Optimization
With recent developments and successes in cell and gene therapy, the biopharmaceutical industry is facing increased demand for safe and efficient delivery systems (1). Viral vectors, including adenoviruses (AV), adenoassociated viruses (AAV), and lentiviruses (LV), are among the most common delivery agents because they infect mammalian cells efficiently. Suspension cultures have become a popular choice for robust and scalable viral manufacturing systems. Using stable cell lines that integrate all or part of the viral production elements adds further benefits by…
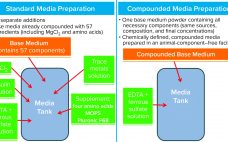
Compounded Media Powder Streamlines Cell Culture Media Preparation Operations
Cell culture medium is critical to cell growth, metabolism, and protein expression. It provides for optimum pH, osmolality, and nutrients in an environment that is essential for cell survival, growth, and expression of proteins and/or metabolites and drug-substance modalities of interest (1). A complete medium typically contains basic nutrients such as carbohydrates, amino acids, lipids, salts, vitamins, trace metals, growth factors/hormones (e.g., insulin), antishear factors, and other chemicals that facilitate cell growth and protein expression and may stabilize recombinant protein…
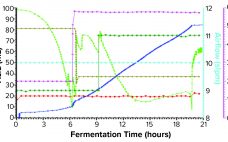
Oxygen Control Strategy and Yield of Recombinant Antibody Fragments Produced in Fermentation
Immunoglobulin molecules are used extensively in therapeutic treatments, diagnostic applications, and fundamental academic research. Traditionally, full-length antibodies and smaller fragments such as the recombinant antigen-binding fragment (rFab) are produced through mammalian cell culture. rFabs also are small enough to be produced in Escherichia coli through fermentation (1, 2). Because disulfide bonds cannot be formed efficiently in the reducing cytoplasm of E. coli, rFabs are supplemented most commonly with a signal sequence that directs them to the more oxidizing bacterial periplasm…
Bioreactor Automation Driven by Real-Time Sensing: Enhancing Productivity Through Accurate, Efficient Glucose Control
In the quest for improved quality and productivity in drug manufacturing, the industry is moving toward increasing use of bioreactor systems with real-time integrated monitoring and advanced analytics that can enable automation, drive performance, and improve data-rich quality control. However, there are multiple options for sensors and technologies that monitor important cell-culture variables or critical process parameters (CPPs). Furthermore, cell culture vessels can be disposable single-use bioreactors (SUB) or reusable glass or stainless-steel models. They can operate in stirred tanks,…
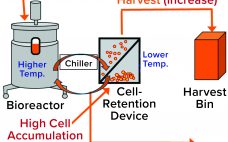
Product Quality Attribute Shifts in Perfusion Systems, Part 2: Elucidating Cellular Mechanisms
Part 1 of this two-part report describes an investigation into the potential cause(s) and ways to control a product quality attribute (PQA) of a protein expressed in perfusion cell culture (1). The presence of low–molecular-weight (LMW) species following size-exclusion high-performance liquid chromatography (SEC-HPLC) is a protein quality attribute that can indicate an increase in truncated forms of the expressed protein and/or other LMW moieties. The expressed protein in this study is a heavily glycosylated recombinant glycoprotein (rGP) comprising two subunits:…
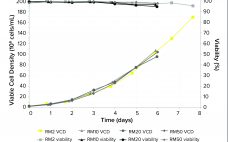
Intensified Seed Train Strategy for Faster, Cost-Effective Scale-Up of Biologics Manufacturing
The high costs of and limits on global accessibility of biologics such as monoclonal antibodies (MAbs) are focusing the biopharmaceutical industry’s attention on strategies for rapid, economical development of such therapies. Process intensification is one approach to help shorten manufacturing timelines and reduce cost of goods (CoG) (1, 2). Today, process intensification in upstream cell culture enables biologics manufacturing in facilities with smaller footprints and lower scale-up volumes than was possible before. Intensified processing of Chinese hamster ovary (CHO) clones…
eBook: Raw Material Control Strategy — Leveraging Knowledge of Material Attributes and Data Analytics as Key Elements
Ensuring pharmaceutical quality begins with in-depth understanding of process/platform capabilities, which is informed by knowledge gained through product and process development, subject-matter expertise, and lessons learned from experience. And all outside factors that can affect manufacturing outcomes must be taken into consideration. Extra vigilance is necessary for understanding potential sources of variation and maintaining robust control strategies to ensure process consistency — and ultimately product quality for patients. Biomanufacturing unit operations require multiple raw materials that must be documented as…