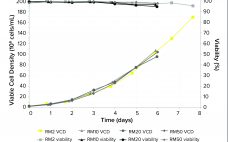
The high costs of and limits on global accessibility of biologics such as monoclonal antibodies (MAbs) are focusing the biopharmaceutical industry’s attention on strategies for rapid, economical development of such therapies. Process intensification is one approach to help shorten manufacturing timelines and reduce cost of goods (CoG) (1, 2). Today, process intensification in upstream cell culture enables biologics manufacturing in facilities with smaller footprints and lower scale-up volumes than was possible before. Intensified processing of Chinese hamster ovary (CHO) clones…
Author Archives: Dominic Grone
Simplifying Progress
Never has there been a more critical time to be a company delivering technologies and expertise that helps get new biologics and vaccines to patients faster. Sartorius is that company. Working in partnership with scientists, we help turn research discoveries into real-world medicines. Our bioprocessing equipment and know-how are called on every day to create biologics that treat debilitating diseases, including cancer and arthritis, and make affordable vaccines to prevent common infections. When global organizations such as the Jenner Institute…
New Single-Use Bioreactor for Cellular Products of Consistent Quality: BIOSTAT® RM TX Bioreactor with Flexsafe® RM TX Bags
Sartorius Stedim Biotech (SSB), a leading international supplier for the biopharmaceutical industry, has launched the BIOSTAT® RM TX single-use bioreactor, a new wave-action mixer system developed specifically for closed, automated expansion of consistent-quality cell therapies such as ex vivo cellular immunotherapies. This new good manufacturing practice (GMP) platform combines SSB’s established single-use Flexsafe® bag technology with the company’s expertise in biopharmaceutical automation. Fill out the form below to read the complete technology review.