Serum is the most commonly used supplement in cell culture. Fetal bovine serum (FBS) is the common choice because it contains high concentrations of growth factors and other important signaling molecules (e.g., adhesion proteins, nutrients, carrier proteins, cytokines, and hormones) required for cell survival and differentiation together with its buffering capabilities. FBS production begins with the collection of whole blood from bovine fetuses under aseptic conditions. Once collected, the blood is allowed to clot, and the serum is mechanically separated.…
Upstream Processing
eBook: Microbial Expression — The Right Choice for Large Peptides and Small Proteins
Although animal cell culture has dominated the biopharmaceutical industry for some years now, microbial expression remains important for producing proteins that don’t require posttranslational modifications — or only those that prokaryotic microbes can perform. It also offers an affordable option for antibody fragments and gene therapies. Microbes may be less fragile than animal cells, and they do require simpler media, but they present other challenges related to temperature management and oxygen transfer in culture. Wherever practical, bacterial expression is preferred…
Implementation of Single-Use Miniature Bioreactors to Support Intensified Cell Culture: Using Functional Performance Indicators to Assess a Small‑Scale Model
Changes to bioprocessing in the biopharmaceutical industry are driven by the need for increased speed, lower cost of goods (CoG), and greater flexibility (1). To meet these challenges, the industry is adopting strategies that include intensified processing. During the initial stages of intensified processing, it is essential to identify the most productive and/or stable clones for use before starting pilot-scale studies. That requires screening large numbers of clones and then further testing the most promising ones in benchtop bioreactors. The…
Improving Bioprocess Expression Systems: A Clean Alternative to CRISPR/Cas9
Chinese hamster ovary (CHO) cells have emerged as a robust platform for bioprocessing serving both early and late-stage biotherapeutic drug supply. However, these cells and other hosts (e.g., HEK293), can be optimized for even greater potential through advanced gene editing. For example, when the endogenous glutamine synthetase (GS) gene is knocked out in CHO cells, a sixfold increase in high-producing cell lines is achieved (1). In another study, CHO with annexin A2 (ANXA2) and cathepsin gene (CTSD) knockouts were introduced…
Matrix: The Highly Automated Multibioreactor Solution That Fits to Your Bench Space, Bioprocessing Needs, and Budget
To improve their bioprocess performance, life-science specialists need flexibility in their R&D laboratories because of constantly changing bioprocessing demands. In addition, more experiments need to be performed with more accuracy and reproducibility on less bench space than ever before — and with limited budgets. Therefore, having flexibility in the number of bioreactors that fit available bench space and budget is crucial — along with the flexibility to connect and integrate the right software, sampling tools, and analytical devices. Running multiple…
Innovative Strategies for Cell Culture Media Preparation
Although the handling and preparation of cell culture media can seem routine, a number of risks are associated with such operations. Identification and mitigation of associated risks can help ensure consistency of performance, minimize likelihood of contamination, and protect employees while enabling greater efficiencies in upstream processes. Here we describe a number of strategies for reducing risks and streamlining media-related workflows. Simplifying Handling of Cell Culture Powders Media preparation typically is quite labor intensive and poses risks related to containment…
eBook: Addressing Production Complexities — Strategies for Working with Difficult and Susceptible Proteins
All proteins are complex — but some are more complex than others, particularly when it comes to recombinant protein expression and production in commercial quantities. What works in a research laboratory to make a milligram of pure protein for study won’t necessarily work on a manufacturing floor to make kilogram batches for drug-product formulation. An increasing number of technological options are available, however, from a simple switch in expression host or adding folding steps in downstream processing to special genetic…
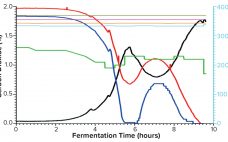
Comparative Study of Single-Use and Reusable Fermentors: Production of Recombinant Proteins Through Bacterial Fermentation
Single-use bioreactors have become widely accepted and well established for cell culture applications in the biopharmaceutical industry for over a decade (1). Abbott Diagnostics has moved into this technology already for commercial production of some biologic molecules. However, single-use systems (SUSs) are rarely available for microbial applications, mostly because of the technical challenge in designing cost-effective SUSs that can meet high oxygen transfer needs and remove excessive heat generated during fermentation. Thus, an important part of our biologics manufacturing —…
The Critical Role of Media in Intensified Upstream Processes
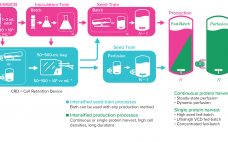
As the need for novel therapeutics increases, so does pressure on the biopharmaceutical industry to improve productivity, accelerate development, increase, and reduce costs — all while ensuring drug product quality. Upstream intensification strategies such as perfusion culture can address those challenges and achieve higher protein titers that can translate into higher throughput, improved flexibility, and compressed timelines. Successful implementation of perfusion culture or the transition to perfusion from fed-batch culture requires a different and strategic approach to media selection, not…
Production of Transient Lentiviral Vectors in HEK 293T Cells: Cultivation on Fibra-Cel Disks in a Single-Use, Packed-Bed, Stirred-Tank Bioreactor
Although demand for lentiviral vectors (LVs) for cell and gene therapy is increasing, the standard two-dimensional culture systems used to produce LVs present significant disadvantages. Current bottlenecks in LV production are caused mainly by such disadvantages. Switching to use of bioreactors can eliminate those problems because bioreactors offer the benefits of process automation, tight regulation of production conditions, and reduced labor input. The study reported herein was carried out by the group of David Parsons at the University of Adelaide.…