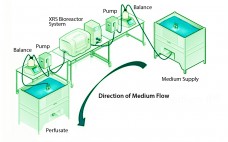
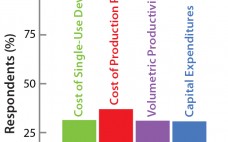
Although implementation of continuous manufacturing for biopharmaceuticals is in the early stages, continuous cell culture technology has been around for close to thirty years. Perfusion was initially developed in the late 1980s as a means for increasing protein titers (1). However, high costs driven by media consumption limited widespread commercial adoption. In the same time frame, advances in cell line engineering, media composition, and bioreactor design led to 10-fold increases in titers for batch and fed-batch modes, eliminating the first…
Upstream Processing
Bioreactor Manufacturing Platforms for Cell Therapies
As an increasing number of cell therapies move into late-phase trials, developers are considering innovative solutions to address scale-up and commercialization challenges. Many of their questions focus on the technologies and engineering strategies that will be needed to optimize their processes, especially bioreactors. At the January 2016 Phacilitate Cell and Gene Therapy World conference, Siddharth Gupta, a scientist at Lonza (Walkersville, MD), talked about the effects of upstream process decisions on product quality in his presentation “Bioreactor Manufacturing Platforms: So…
Orbital Shaking and Acoustic-Resonance Mixing: Comparing Culture Characteristics
Production of recombinant proteins usually happens in suspension cultures, with oxygen limitation playing a major role. Oxygen and nutrition feeds are of great significance to aerobic suspension cultures. Oxygen is often the controlling factor in orbital shaken systems because oxygen transfer occurs only through diffusion, which is limited by gas-exchange surface and mixing characteristics. Here, we compare growth characteristics of microbial cultures in a standard shaken incubator with those of cultures in a RAMbio fermentation system, paying particular attention to…
Development, Qualification, and Application of a Bioreactor Scale-Down Process: Modeling Large-Scale Microcarrier Perfusion Cell Culture
Qualified scale-down models of large-scale cell culture processes are essential to conducting studies for applications such as investigating manufacturing deviations, enhancing process understanding, and improving process robustness. For example, scale-down models can be used for raw material investigations as well as evaluation and qualification of new good manufacturing practice (GMP) cell banks for manufacturing implementation. Process characterization studies are performed also with qualified scale-down models to improve process consistency (1, 2). Often it is impractical to conduct investigational studies at…
Heading for a CHO Revolution: The Need for Cell Line Engineering to Improve Manufacturing Cell Lines
The first recombinant protein licensed for use by the United States Food and Drug Administration (US FDA) was human insulin in 1982 (1). That approval was followed in 1987 by the development of tissue plasminogen activator (tPA), the first complex glycosylated protein generated in mammalian cells to be licensed for therapeutic use. Since then, this area of biology has rapidly expanded in clinics: The FDA approved an average of 15 new biological entities every year between 2006 and 2011 (2).…
Ask the Expert: FOLDTEC Refolding of Biopharmaceuticals A Case Study of Recombinant Thrombin
with Dr. Andreas Anton and Dr. Sebastian Schuck Poorly soluble substances form aggregated inclusion bodies (IBs) in microbial cells containing incorrectly and/or incompletely folded target proteins. Wacker Biotech, a full-service contract manufacturer of biopharmaceuticals based on microbial systems, has introduced FOLDTEC refolding technology for bioengineered therapeutic proteins. The proprietary platform uses specifically developed and optimized bacterial strains and a patented, antibiotic-free expression system. In a BPI webinar on 9 November 2015, Wacker’s director of bioprocess development (Andreas Anton) and head…
From Chips to CHO Cells: IT Advances in Upstream Bioprocessing
Advances in our capabilities for data acquisition, storage, and manipulation are providing the biopharmaceutical industry with an increased understanding of what must be controlled in bioproduction as well as the ability to control it. Developments in hardware, processing algorithms, and software are changing the landscape of bioprocess administration. Increased power for information gathering and processing began with the remarkable increases in microprocessor speed, pipelining, and parallelism over the past couple of decades (1); it continues with advances in data handling…
Optimizing Cell Culture Productivity: New Findings on the Impact of Recombinant Protein Supplements
This webcast features: Harris Grevelis, Product Manager for Upstream Technologies at Repligen Cell culture supplements are essential for long-term growth and productivity of cell lines in serum-free media formulations. Both insulin and LONG®R3 IGF-I, a more potent supplement that directly targets and activates the IGF-I receptor, are employed by the biopharmaceutical industry today to support the growth of recombinant cell lines. This webinar will show how these two supplements, used separately and in combination, impact viable cell density and IgG…
Bioreactor Design for Adherent Cell Culture: The Bolt-On Bioreactor Project, Part 4 — Process Economics
The Bolt-on Bioreactor (BoB) project is an independent initiative developing and commercializing a bioreactor for efficient, automated culture of adherent cells for biopharmaceutical applications (1). After conducting thorough research on available culture systems for adherent cells, the BoB team believes that a successful alternative to existing devices must solve four major challenges: volumetric productivity (2), process automation (3), containment and sterility (4), and process economics. This month concludes a four-part series addressing each of those challenges while describing design features…
Fluid Dynamics of a Single-Use, Stirred-Tank Bioreactor for Mammalian Cell Culture
The benefits of single-use technologies in both upstream and downstream operations are now widely acknowledged by the biopharmaceutical industry, and have led to radical changes in the design and operation of many bioprocesses. Those changes typically provide more robust processes and increased production flexibility. For mammalian cell culture, cleanable multiuse glass or stainless steel stirred-tank reactors (STRs) have been used successfully for growth of suspension-adapted cell lines in both small- and large-scale systems. However, achieving the same or better performance…