Resolving the COVID-19 pandemic depends on treatments, testing, and ultimately a widely disseminated vaccine against SARS-CoV-2. In recent decades, the biopharmaceutical industry has developed new approaches to vaccination using antigens, virus-like particles (VLPs), viral and bacterial vectors, and nucleic acids. Current events have placed those innovations at the front and center of public attention, offering many companies an opportunity to demonstrate their potential in an unprecedented way. Here, BPI’s senior technical editor describes the challenges that developers face in doing…
Manufacturing
Developing Process Control Strategies for Continuous Bioprocesses
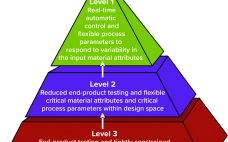
Process control enables biomanufacturers to ensure that operating parameters are within defined specifications. A control strategy should be established during early stages of process development while process and product performance are being defined using risk-based methods such as quality by design (QbD) and process analytical technologies (PATs). Confirming process control as an essential part of product development creates greater process knowledge and understanding and provides the first steps toward process optimization. By understanding how process performance relates to product quality,…
eBook: A Light-Chain Platform for Developing Bispecific Antibodies
Biopharmaceutical researchers are focusing on novel platforms to quickly develop bispecific antibody (BsAb) therapies for treating complex diseases such as cancer. BsAb therapies offer several advantages because they are designed to bind two unique epitopes. In this report, Bill Lundberg, president and chief executive officer at Merus, describes his company’s efficient approach to developing BsAbs using “common light chain” technology and other proprietary strategies. Lundberg also addresses the company’s approach to typical BsAb development and manufacturing challenges. Fill out the…
Bioprocessing 4.0 Accelerates Biological Research and Development Using Computer-Aided Biology
Computer-aided biology describes a growing ecosystem of tools that augment human capabilities in the laboratory. In this report we give two case study examples of how computer-aided biology has transformed industrial gene therapy bioprocessing. Read on to discover how Synthace’s Antha cloud-based software platform has enabled industrial collaborators Oxford Biomedica and the Cell and Gene Therapy Catapult to harness the power of Bioprocessing 4.0 by: incorporating new process analytical technologies (PAT), such as Raman Spectroscopy, into their unit operations automating…
Discussions at Phacilitate 2020 on Business, Manufacturing, and Future Trends
Presenters in the three main program tracks at the Phacilitate Leaders World conference in Miami, FL, this past January represented sponsor-developers of cell/gene-therapy (CGT) products, contract service providers, and technology suppliers to the industry. Topics include process and product development strategies for advanced therapies, regulatory and inspector expectations, automation and closed-system processing, the choice between in-house and outsourced manufacturing, quality assurance and control, analytical methods, viral vectors, and artificial intelligence and Industry 4.0. At the end of each session, presenters…
The Technology of Tomorrow — Today
Sponsored by BioProcess International and its sister publication BioProcess Insider, the “Tech of Tomorrow Zone” at Phacilitate 2020 played host to a number of companies showcasing platforms and ideas that they believe can revolutionize cell and gene therapy (CGT) manufacturing. Some common themes arose in this diverse zone, highlighting technologies from stem-cell supply solutions to viral-vector filling. Participating companies are aware of the complexities involved in producing regenerative medicines, and each proposed solution was intended to reduce the burden on…
Fighting Alzheimer’s Disease with a Breakthrough Peptide
Even more so than cancer, Alzheimer’s disease is one disease that many people fear greatly. The thought of falling prey to the inevitable desecration of our own minds is something that makes even the bravest among us shudder. If we’re robbed of our sense of who we really are, we imagine, then we are doomed to live our last days without the dignity that defines us and that we hold most dear. The ultimate horror of the condition is that…
Shared Clean-in-Place Systems: To Share or Not to Share?
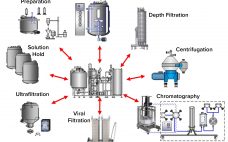
Risk of viral contamination is a an accepted part of developing biopharmaceutical products derived from mammalian-cell culture. Viral safety is achieved through a combination of complementary approaches such as selecting non–animal-derived raw materials, testing cell banks, testing for adventitious virus contamination during cultivation, and demonstrating viral reduction capacity of a purification process (1). The latter commonly is referred to as viral clearance by orthogonal purification. Clearly, viral clearance and appropriate viral segregation are important considerations in biopharmaceutical manufacturing process and…
New Directions in Bioprocess Development and Manufacturing
The biopharmaceutical industry continues to grow at a rapid pace. That growth is accelerating even more because of keen investor and research interests in cell and gene therapies. My focus below is on several key growth areas in the field, specifically aspects of development and biomanufacturing. This discussion builds on my 2018 column on top trends in bioprocessing (1). In that article, I identified five key trends that continue to resonate now: rapid development of high-productivity upstream processes advances in…
Scalable Manufacturing of Lentiviral Gene Therapies
Lysosomal storage diseases are caused by mutated genes that express defective lysosomal proteins, such as essential enzymes. For example, cystinosis is a metabolic disease caused by a defective gene that encodes cystinosin, an exporter protein (Figure 1). Avrobio develops gene therapies to treat lysosomal storage diseases. On 8 October 2019, the company announced that its first patient had been dosed in the AVR-RD-04 investigational gene therapy program, which involves genetic modification of the patient’s own hematopoietic stem cells to treat cystinosis.…