Hitachi Chemical will add two production plants and 600m2 of cleanroom through the acquisition of German cell therapy manufacturing firm apceth Biopharma. The deal, expected to close in April, sees Japanese firm Hitachi acquire all shares of apceth for JPY 9.4 billion ($86 million) from Santo Holding GmbH, FCP Biotech Holding GmbH and other individual shareholders. apceth has around 120 employees operating from its site in Munich, Germany. The site includes two GMP/BSL2 cell and gene therapy production facilities, including…
Search Results for: regenerative medicine
Finding closure: Lowering the costs of cell and gene therapies
Fully closed and automated platforms are key to reducing the current high costs of cell and gene therapy manufacturing, say industry experts. Cell and gene therapies (CGTs) have come a long way over the past two years, resulting in two chimeric antigen receptor (CAR) T-cell therapies â Kymriah and Yescarta â and the novel gene therapy Luxturna reaching commercialization. But now these therapies have proved themselves, the major talking point among delegates at the Phacilitate conference in Miami, Florida last…
Phacilitate: Cell & gene industry flocks to Miami
The cell and gene therapy industry congregated in Miami to discuss supply chain strategies, regulatory rationale and the high manufacturing costs of advanced therapies. BioProcess Insider attended the Phacilitate Leaders World Summit, co-located with the World Stem Cell Summit, in Miami, Florida last week. The conference reflected on the first full year where three advanced therapies – Kymriah, Yescarta, and Luxturna – proved themselves on the commercial stage, heralding in a new frontier for regenerative medicines. But industry quickly rose above…
Manufacturing costs the âbiggest threatâ to cell and gene therapies
This is the first time in history that we can make more effective medicines than we can afford, says Dark Horse Consulting. The Phacilitate conference took place in Miami this week, and in the plenary session Anthony Davies, founder of cell and gene therapy specialist firm Dark Horse Consulting, described the latest developments in the field of regenerative medicine. With the approvals of Kymriah (tisagenlecleucel), Yescarta (axicabtagene ciloleucel) and Luxturna (voretigene neparvovec) in 2017, âthis field finally had the year…
Cell and gene therapies: FDA expects 10 to 20 approvals per year by 2025
The US FDA predicts it will receive more than 200 regenerative INDs per year from 2020 and will add 50 additional staff to review these products. There has been âa large upswing in the number of investigational new drug (IND) applicationsâ for cell and gene therapies received by the US Food and Drug Administration (FDA), commissioner Scott Gottlieb and Peter Marks, director of the Center for Biologics Evaluation and Research (CBER) said in a statement yesterday. The Agency has so…
Business must harness tech and innovation to thrive, says new Locate Bio CEO
Biotech must work at the frontiers of biology and innovation while operating in a highly regulated environment says business development expert Nick Staples, the new CEO of Locate Bio. UK-based cell and gene therapy firm Locate Bio has had a reshuffle at the top, hiring veteran biopharma business executive and industry analyst Dr Nick Staples as CEO. BioProcess Insider spoke with Staples to find out his vision for Locate Bio, and how best to manage the often conflicting business and…
Standardizing Human MSCs As Critical Raw Materials in Cell Therapy Products: Streamlining Clinical Translation
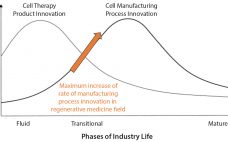
Advancements in cell therapy, biofabrication, and synthetic biology have driven the growth of the global regenerative medicine (RegenMed) industry in the past decade. The industry has developed innovative treatment options for patients with otherwise unmet medical needs (1). Human or animal cells or tissues are used as critical raw materials in cell therapy products that can replace, regenerate, or augment patientsâ diseased, dysfunctional, or injured cells, tissues, or organs. These cells or tissues can be unmanipulated, or their biological characteristics…
A Multistep Research Protocol to Develop and Implement Validated Guidelines for CMO RFI and RFP Processes: Biopharmaceutical Vendor Evaluation and Selection Minimum Standards (BioVesel)
Pursuant to the proposal for validated minimum standards for biopharmaceutical contract manufacturing organization (CMO) request-for-information (RFI) and request-for-proposal (RFP) processes (biopharmaceutical vendor evaluation and selection minimum standards, BioVesel) (1), we propose herein a multistep research protocol to develop and implement the BioVesel standards. This proposal is intended as a basis for discussion among mulitple stakeholders. Detailed research protocols for each proposed stage in the development and implementation of BioVesel will be drafted and published separately. The context of the proposed…
Rolling with the âTides: Elucidating the Role of Peptides and Oligonucleotides in the Biopharmaceutical Industry
In earlier issues of BPI we published a few âElucidationâ closers that we called âDefining Moments.â Since then, we have tried to distinguish key confusable terms from one another. Those presented (and sometimes âelucidatedâ) have been analytical and bioanalytical, spectroscopy and spectrometry, and biosimilars and biobetters. They are just a few of the many confusable terms in the biopharmaceutical industry. For example, when someone says âdrug delivery,â a formulator will think of a syringe or transdermal patch, but a logistics…
The Need for Adherent Cell Manufacturing: Production Platform and Media Strategies Drive Cell Production Economics
Most commercial biopharmaceuticals originated from academic research laboratories and start-up development laboratories. Despite such products having differences in modalities and targeted disease indications, and whether their target patient populations are relatively small or approaching blockbuster status, at a key point in development, biopharmaceutical production must scale up from laboratory to commercial production. That movement from research to development and then to manufacturing forces attention on economics and speed to market, and it drives innovative approaches to producing biopharmaceutical cell compositions…