The successful commercialization of a biopharmaceutical product begins with a robust and productive cell line. Inefficient cell-line development (CLD) can lead to costly delays and roadblocks. For that reason, small, new, and virtual companies — and even established and mid-size companies — often seek the support of outsourcing partners to develop their cell lines. Outsourcing CLD activities can ease many pressures associated with manufacturing new biotherapeutics. The benefits of outsourcing CLD and associated processes include access to specialized expertise and…
Manufacturing
Reducing Cell and Gene Therapy Development Time and Cost with New Purification Strategies
The past 40 years have ushered in the most advanced medicines the world has ever seen, with tremendous improvements in biomanufacturing technologies to enable their development. Advances in production technology have brought significant improvements in upstream productivity, which then caused bottlenecks in downstream processing. Although many bottlenecks have been resolved for most biologics, new modalities such as gene therapies and mRNA vaccines are driving the need for differentiated purification solutions. Meanwhile, pressures to increase efficiency and reduce costs continue to…
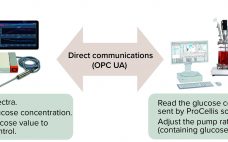
Seamless Integration of Glucose Control: Using Raman Spectroscopy in CHO Cell Culture
The process analytical technology (PAT) and quality by design (QbD) guidelines promoted by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) support the idea that quality cannot be “tested into” a biologic product but must instead be part of its process design. Seamless integration of analytical data with bioprocess monitoring and control is crucial to understanding a process and overcoming manufacturing challenges that arise in the course of development. Monitoring of product quality attributes (PQAs)…
eBook: Cell and Gene Therapies — Optimization Strategies for Processing and Potency
Although relatively new to the biopharmaceutical industry, cell and gene therapy development and manufacturing are advancing rapidly. At Informa Connect’s September 2021 Cell & Gene Manufacturing & Commercialization Conference and Exhibition, held in Boston and online, presentations reviewed concerns that arise when processing complex therapies and highlighted some innovative strategies for surmounting those obstacles. Most of those approaches described during the event used data-driven solutions, with each step building on the information gained from the previous one. High-throughput technology platforms…
eBook: Vaccines Revisited — The Past, Present, and Future of Nucleic-Acid Vaccine Production
The quarterly BioProcess Insider eBook series launched in 2021 to investigate specific modalities and business strategies driving the biopharmaceutical sector based upon trends and discussion points presented in the Insider’s pages. Two of the four quarterly publications — this one included — have focused on the vaccine industry. If the series had launched in 2019, cell and gene therapies might have warranted a double edition, or perhaps investments in antibodies and antibody fragments would have driven a bispecific focus. Five…
Optimizing the AAV Transfection Process in Suspension Cells
Given the potentially curative nature of gene and gene-modified cell therapies and the successful launches of several such products over the past five years, markets and investments are growing significantly in the sector. In the United States alone, the US Food and Drug Administration has granted approval for several genetic therapies, including Strimvelis (autologous CD34+, developed by GlaxoSmithKline and later sold to Orchard Therapeutics) in 2016 Luxturna (voretigene neparvovec, Spark Therapeutics), Yescarta (axicabtagene ciloleucel, Kite Pharma/Gilead), and Kymriah (tisagenlecleucel, Novartis)…
Viral Safety of Viral Vectors:
Special Concerns Arise When the Virus Is the Product
As anyone who has focused on host-cell proteins as process contaminants can tell you, trying to purify a specific type of molecule from a large mixture of many similar molecules is like trying to find a few particular needles in a huge pile of varied needles. The same could be said for purifying viral vectors from cell culture fluids. When viruses are the products, unwanted viruses are contaminants that must be separated away — or better yet, prevented from being…
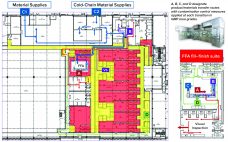
Formulation, Fill and Finish of Lentiviral Vectors: Part 1 — Case Study in Facility and Process Design
Over the past few years, Oxford Biomedica Ltd. (OXB) has developed and implemented a fill–finish platform (“Oxbox,” Figure 1) at its viral vector processing facility in the United Kingdom. The facility includes four segregated bulk viral-vector drug substance (VS) suites, where closed systems and bioburden control processes apply, and two viral-vector drug product (VP) fill–finish suites that apply aseptic processing, with space for expansion by scale-out as product output demand increases. Segregated suites enable the facility to process different viral…
Spurring on Innovation in Gene Therapy Development
Gene therapies based on adenoassociated virus (AAV) vectors hold promise for treating myriad conditions. Immunogenicity remains a challenge for such products, however. With support from PerkinElmer, Roland W. Herzog (professor of pediatrics and Riley Children’s Foundation professor of immunology at the Indiana University School of Medicine) joined Nagendra Venkata Chemuturi (scientific director of global research for drug metabolism and pharmacokinetics, DMPK, at Takeda Pharmaceuticals) to deliver a BPI “Ask the Expert” presentation exploring strategies for minimizing immune responses to AAV-based…
eBook: Speed to IND — Practical Wisdom for Uncertain Times
Even in “normal” times, companies need to balance time to filing an investigational new drug (IND) application against careful consideration of processes that can have far-reaching consequences on the quality of biologic products. But supply-chain interruptions still feature prominently in the news during the ongoing COVID-19 pandemic. From equipment to chemicals to plastic components, end users, their suppliers, and (critically) their suppliers’ suppliers all are feeling growing uncertainty about production timelines and availability of materials. In this eBook, BPI’s editor…