Seven years ago, the US Food and Drug Administration (FDA) approved the first product in a new class of biologics: antibody–drug conjugates (ADCs). The idea for these products already had been hatched a decade earlier when the promising field of antibody research — touting such molecules as “magic bullets” — had faltered, specifically against oncology-related indications. The early crop of anticancer monoclonal antibodies (MAbs) proved to have only limited efficacy, and interest in developing antibodies as therapeutic agents against cancer…
Emerging Therapeutics
Process Analytics and Intermediate Purification of Bispecific Antibodies with a Non-Affinity Platform
The therapeutic benefits of monoclonal antibodies (MAbs) have been demonstrated in recent decades with uncontestable success as treatments for human disease. Despite MAbs’ key features such as specificity, selectivity, and safety, the format has limitations (1, 2). Bispecific antibodies may overcome number of difficulties (3). Multiple formats of bispecific antibodies have been developed, although only the κλ-body is fully human and devoid of linkers or mutations. It requires no genetic modifications of heavy and light chains and results in bispecific antibodies…
Therapeutic Modalities: Business and Manufacturing Strategies Influencing Decisions to Develop One Therapy Type Rather Than Another
Moderator Patricia Seymour, with John Lee, Michael Kaufman, Jennifer Michaelson, and Weichang Zhou Following introductions of the panelists and their companies’ technologies, moderator Patricia Seymour began the discussion about challenges related to choosing different modalities and addressing related manufacturing concerns. Targeting Modalities Michaelson began by describing how Cullinan Oncology selects its targets and modalities, how it approaches those early phase decisions, and what its primary driver is to get into the clinic as quickly as possible. She talked about challenges…
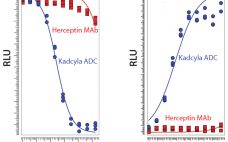
The Development of a Next Generation Antibody–Drug Conjugate (ADC)
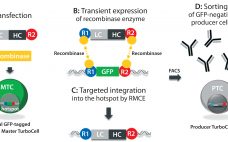
Michelle Zengh, chief operating officer, MabPlex International Company Ltd. MabPlex presents a complete solution from gene cloning to antibody–drug conjugate (ADC) fill–finish with a timeline from gene cloning to an investigational new drug application (IND) in 22 months. The company has facilities in San Diego and in China. It can perform cloning and high-yield stable cell-line development, process development and scale-up, CGMP quality and kilogram-scale monoclonal antibody (MAb) and payload manufacturing, large-scale conjugation (150–500 L), large-scale purification, formulation, and fill–finish.…
eBook: Manufacturing CAR-T Cell Therapies — Insights and Challenges
The rapid evolution and clinical success of T-cell immunotherapies is an exciting advance in the war on cancer. This treatment modality uses engineered cells from a patient’s own immune system to target and destroy cancerous cells. Chimeric antigen receptor T-cell (CAR-T) therapy is emerging as the most studied treatment in T-cell immunotherapy and is the basis for many ongoing clinical trials. FDA approval of the first two CAR-T therapies in 2017 provides a regulatory development framework, but optimization of CAR-T…
eBook: Innovations in CRISPR Technology — A Perspective on Research and Bioprocess Applications
One of the fastest growing areas in genome engineering is research using the powerful editing tool of clustered regularly interspaced short palindromic repeats (CRISPR). When paired with the Cas9 (CRISPR-associated protein 9), an RNA-guided DNA endonuclease enzyme from Streptococcus pyogenes, the site-specific prokaryotic immune system can be used to cut and manipulate DNA strands in cells of patients with genetic diseases to treat, or in some cases, prevent such diseases. Within the past couple of years, CRISPR has been shown…
Therapeutic IgG-Like Bispecific Antibodies: Modular Versatility and Manufacturing Challenges, Part 2
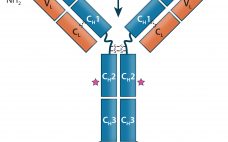
Monoclonal antibodies (MAbs) are bivalent and monospecific, with two antigen-binding arms that both recognize the same epitope. Bispecific and multispecific antibodies, collectively referred to herein as bispecific antibodies (bsAbs), can have two or more antigen-binding sites, which are capable of recognizing and binding two or more unique epitopes. Based on their structure, bsAbs can be divided into two broad subgroups: IgG-like bsAbs and non–IgG-like bsAbs. We have chosen to focus on the former in this review. Part one provides a…
eBook: Bioinks for Bioprinting — Three-Dimensional Printing in Research and Medicine
Three-dimensional (3D) printing is one method of digital biomanufacturing for both basic biological research and translational, clinical applications. The medical field has used it to create such constructions as 3D surgical models for preoperative planning, to assist surgeons in their procedure preparations, which improves postsurgical outcomes. Examples here include generation of cleft-palate models (1), orthopedic applications (2), and cardiovascular surgical planning (3). Other forms of 3D printing for biological applications — such as 3D bioprinting — go beyond such surgical…
Therapeutic IgG-Like Bispecific Antibodies: Modular Versatility and Manufacturing Challenges, Part 1
Antibody-based immunotherapy has advanced significantly since 1986, when the US Food and Drug Administration (FDA) approved the first mouse monoclonal antibody (MAb) for clinical use: Orthoclone OKT-3 (muromonab-CD3). In the intervening years, researchers have applied the tools of genetic engineering to clone immunoglobulin G (IgG) genes into a number of expression vectors. In the 1990s, the bioprocess industry was able to produce fully human antibodies in cultured cells. As of June 2017, the FDA and the European Medicines Agency (EMA)…
Antibody–Drug Conjugates: Fast-Track Development from Gene to Product
In the fight against cancer, antibody–drug conjugates (ADCs) represent an increasingly important therapeutic approach. These biopharmaceuticals are designed to maximize the therapeutic index of cytotoxic small-molecule drugs through their selective delivery to tumor cells while leaving normal, healthy cells untouched. Structurally, an ADC is a monoclonal antibody (MAb) conjugated by a chemical linker to a potent cytotoxic drug. Conceptually, the MAb serves as the delivery component, targeting a specific tumor antigen that ideally is not expressed (or is expressed at…