Recent advances in molecular biology are expediting genomic sequencing to underpin precision medicine. Such progress is positioning gene and gene-modified cell therapy on the cusp of an extraordinary revolution in patient care for presently unmet medical needs — and a new therapeutic class that could rival monoclonal antibodies (MAbs) in importance. However, despite substantial strides made in clinical trials, the bioprocessing community is struggling to fulfill growing demands for biomanufacturing capacity to make gene and gene-modified cell therapies — including…
Emerging Therapeutics
Automation of CAR-T Cell Adoptive Immunotherapy Bioprocessing: Technology Opportunities to Debottleneck Manufacturing
Continued clinical efficacy demonstrations of cell-based immunotherapies (iTx) such as chimeric antigen receptor T cell (CAR-T) therapies has made the prospect increasingly likely of an immunotherapy product achieving conditional market authorization in the short term. For example, Novartis and the University of Pennsylvania’s lead candidate (CTL019) for treating a range of hematological malignancies received breakthrough status from the US Food and Drug Administration (FDA) in 2014, permitting access to an expedited drug development pathway for high unmet medical needs (1).…
Uniting Small-Molecule and Biologic Drug Perspectives: Analytical Characterization and Regulatory Considerations for Antibody–Drug Conjugates
Cosponsored by CASSS (an international separation science society) and the US Food and Drug Administration (FDA), the January 2010 CMC Strategy Forum explored antibody–drug conjugates (ADCs), which are monoclonal antibodies (MAbs) coupled to cytotoxic agents. The ADC platform of products is being used more and more for clinical evaluation in oncology. More than a dozen companies are developing several types, including products conjugated with calicheamicin, auristatins, and maytansinoids. Such products use the specificity of a MAb to deliver a cytotoxic…
Fine-Tuning ADCs for Best-in-Class Therapeutics
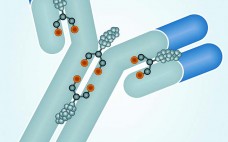
Antibody–drug conjugates (ADCs) use the targeting ability of a monoclonal antibody (MAb) to deliver a highly biologically active drug to diseased cells while sparing healthy cells, creating potent and effective therapies. This emerging class of novel drugs currently focuses almost exclusively on cancer treatment. Two blockbuster ADCs — brentuximab vedotin (Adcetris from Seattle Genetics) for treatment of rare lymphomas and ado-trastuzumab emtansine (Kadcyla from Genentech/ Roche, manufactured by Lonza) for treatment of HER2-positive metastatic breast cancer — have improved treatment…
Predicting Aggregation Propensity and Monitoring Aggregates in ADCs
Antibody–drug conjugates (ADCs) are monoclonal antibodies coupled to cytotoxic agents with stable linkers. ADCs travel to target cells, where the antibody binds to its antigen expressed on the cell surface. Upon binding, the full ADC can be internalized by a process called receptor-mediated endocytosis. That process is followed by lysosomal degradation of ADC complexes, which ultimately leads to release of the cytotoxic agent and apoptosis of the target cell. Drugs used in ADCs can be up to a thousand times…
Technology Advances Enable Creation of Better ADCs
Antibody–drug conjugates (ADCs) for treatment of cancer combine the tumor-targeting properties of antibodies with the cell-killing properties of cytotoxic drugs. By targeting a drug to a tumor, it is possible to reduce systemic toxicity and thereby enable administration of drugs that are otherwise too toxic to be effective therapies. Although the concept of an ADC is simple, in reality developing an effective treatment is somewhat more challenging. Whether an ADC has sufficient efficacy at a tolerable dose depends on four…
Using a CMO for Your ADC: Access Analytical and Manufacturing Platforms, Specialized Facilities, and Expertise
[Audio Recording] Antibody–drug conjugates (ADCs) are an exciting new area of therapeutics. They bring the “magic bullet” that was promised by Paul Ehrlich over a hundred years ago to reality by targeting cancer cells to deliver chemotherapies without poisoning a patient’s whole body. ADCs offer a promising form of therapy by providing higher safety margins than traditional chemotherapeutics alone, and they make selectivity possible. We should be able to personalize a therapy to the specific cancer expressed in a given…
The Future Directions of ADC Development
This podcast features: David Rabuka, PhD, President, Chief Scientific Officer, Founder, Redwood Bioscience This podcast was recorded based on a presentation given at the 2014 BioProcess Theater which took place at the 2014 BIO International Convention, June 23-26, 2014. The BioProcess Theater, a 50-seat amphitheater located on the exhibition floor at the heart of the BioProcess Zone, provided attendees with daily, complimentary opportunities to listen, learn, and interact with leading experts as they presented, discussed and debated the latest business…