Although today’s vaccines are safer, more effective, and more accessible than they were even 20 years ago, the emergence of new, complex pathogens has exposed limitations in traditional vaccine strategies. Viral vector vaccines (VVVs) hold great promise for confronting those now-intractable pathogens. Combining the best features of live-attenuated and DNA-vaccine approaches, these next-generation prophylactics seek to harness the infectivity of non- or low-immunogenicity viruses to shuttle antigen-encoding DNA from target pathogens into host cells. The resulting transduced cells then initiate…
Emerging Therapeutics
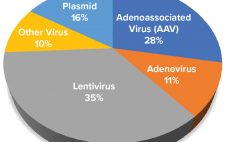
Capacity Analysis for Viral Vector Manufacturing: Is There Enough?
Advanced therapy medicinal products (ATMPs) are engineered to replace defective, disease-causing genes to compensate directly for a genetic defect or to encode a therapeutic protein construct (e.g., chimeric antigen receptor, CAR) for disease treatment. In most instances, a viral vector delivers the engineered genetic payload, targeting cells in situ or ex vivo through cellular modification, expansion, and infusion into a patient. Clinical successes of ATMPs bolstered by regulatory approval of products such as Luxturna (voretigene neparvovec-rzyl, Spark Therapeutics), Kymriah (tisagenlecleucel,…
Analytical Testing Strategies for CAR T-Cell Products
Assay lifecycle development for traditional biopharmaceuticals such as vaccines and monoclonal antibodies (MAbs) has a clearly defined pathway, from preclinical method selection, development, and optimization through the milestones in preclinical phase trials, and finally to postlicensure method evaluations, comparability, and improvements. The analytical development roadmap for nontraditional biologics such as chimeric antigen receptor (CAR) T-cell therapies and gene therapies are not as clearly defined and can present many challenges along the way. Understanding the “what, how, and when” of analytical…
AAV Vector Manufacturing Platform Selection and Product Development
Adenoassociated virus (AAV) vectors have emerged as the prominent delivery mechanisms of corrective gene therapies. Three such products — Glybera (alipogene tiparvovec, uniQure), Luxturna (voretigene neparvovec-rzyl, Spark Therapeutics), and Zolgensma (onasemnogene abeparvovec-xioi, AveXis) — have been licensed, and a growing number of candidates are entering late-stage development. In mapping out an AAV gene therapy product development strategy, biomanufacturers should address fundamental considerations for their manufacturing strategies for both phase 1–2 clinical evaluation and translation for commercial market supply. A manufacturing…
Bioprinting Capabilities and Futures
Bioprinting has advanced rapidly through engineering step changes in the use of three-dimensional (3D) printing. With these developments, living cells can be positioned layer by layer to produce functional tissue structures. Key attributes of this emerging technology are its high scalability and modularity, which enable automated and repeatable manufacture of a wide variety of tissues. These high-throughput biofabrication capabilities equip companies with tools to develop 3D-printed tissues for broad applications, from in vitro drug testing models to therapeutic tissue implants,…
Emerging Treatments for Spinal Cord Damage
Injuries to the spinal cord can cause permanent paralysis and even lead to death, with little or no hope for patients to regain lost function after such trauma has occurred. News of my spinal cord research first came to prominence in the 1980s with a front-page story that chronicles a presentation at the annual Society for Neuroscience meeting (1). That reported the first time that crushed peripheral nerves had been regenerated back into a patient’s spinal cord. Regeneration of spinal…
Formulation Development of Microbiome Therapeutics Localized in the Intestines
Scientists have discovered that microorganisms associated with the body play a critical role in human health (1). The intestinal microbiome is the collection of all combined genetic material of microorganisms in the intestines. An intestinal microbiota, a term frequently used interchangeably with microbiome, is the collection of such microorganisms in the intestine. With the volume of research into the human microbiome expanding, so too is the number of examples of its benefits to our health (1). Like diseases of the…
Immunotherapy: Taking Aim at Solid Cancers
As cell and gene therapies arrive on the market, all eyes have focused on autologous chimeric antigen receptor (CAR) T – cell therapies. At the 2019 Phacilitate Leaders World and Stem Cell Summit in Miami, FL, delegates looked at where the biopharmaceutical industry is going in the cellular immunotherapy space. Whether for off-the-shelf CAR T-cell products, personalized cancer vaccines, or modified natural killer (NK) cells derived from human induced pluripotent stem cells (iPSCs) — cell and gene therapy development is…
Building Toward Antibody–Drug Conjugate Success
“We are witnessing one of the most significant paradigm changes in oncology drug development, with some new types of immunooncology compounds inducing unprecedented increases in survival in certain solid and liquid tumor indications.” —Jagath Reddy Junutula (Cellerant Therapeutics) and Hans-Peter Gerber (Pfizer) (1) An antibody–drug conjugate (ADC) for cancer treatment needs four things to succeed clinically: It needs to target the right antigen using the right antibody, with the most powerful cytotoxin attached by best linker option. These features combine…
Antibody–Drug Conjugate News: From BioProcess Insider
The following news items have appeared on the BioProcess Insider site over the past year. Together they indicate the direction in which the ADC sector is moving. BEYOND ADCETRIS — SEATTLE GENETICS AIMS FOR BIG BIOPHARMA STATUS 30 April 2018: Seattle Genetics is building toward a bigger future. For the first quarter 2018, the company’s total revenues grew to US$141 million (€116 million) compared with $109 million in the same period last year. This was attributed to a 36% increase…