Single-use fermentors (SUFs) offer dramatic advantages over traditional stainless-steel clean-in-place (CIP)/sterilization-in-place (SIP) fermentors. Single-use technology eliminates the need for steam, chemicals, and water to clean and sterilize stainless vessels, which provides a direct reduction in capital cost and environmental-impact mitigation. Single-use platforms also increase equipment and process flexibility significantly, making it possible to switch campaigns from one product to another in minimal time, while eliminating cleaning and associated validation steps (1). Both Cytiva and Thermo Fisher Scientific offer SUFs that mimic the principles of historical stainless-steel systems (2, 3). We have evaluated SUF equipment from both vendors, reporting our evaluation of Xcellerex XDR-50 SUF and HyPerforma S.U.F. 30 units compared with a Sartorius SIP fermentor at a working volume of 10 L (4, 5). In that report, Escherichia coli batches grew to low-level optical densities (20–30 OD600) that are sufficient to generate enough recombinant proteins for many diagnostic assays. Some biologic products require high-density fermentation (>100 OD600) to meet large annual demands or to compensate for low expression levels. Meeting the high oxygen-transfer requirements that many microbial fermentation processes require is a key difficulty in SUF development (6), even more so at higher densities. In our previous publication, we did not discuss how SUFs would perform in such processes.
Recombinant antigen-binding fragments (rFabs) are used widely in therapeutic treatments, diagnostics, and fundamental academic research applications (7, 8). Relatively low yields are a major concern with expression of correctly folded, functional rFabs in E. coli (9). We developed a low-expression rFab process through high-density fermentation using an Applikon eZ-Control SIP fermentor. In that process, rFab yield is influenced by not only the level of aeration, but also the method for control of optimal dissolved oxygen (DO) (10). Further work was required to adapt the process for a Thermo Scientific HyPerforma S.U.F. 30-L fermentor. Here, we report the findings of that work and compare the SUF performance with that of SIP fermentors.
Materials and Methods
Bacterial Strain: We used an E. coli host strain with plasmids controlling expression of the target protein. Expression was induced by adding sterile solutions of isopropyl-β-d-thiogalactopyranoside (IPTG) and arabinose from MilliporeSigma, with final concentrations of 1.0 mM and 12.0 mM, respectively.
Composition of Complex Medium: The composition and preparation of batch and feed media were based on published data (9). The feed medium contains only a carbon source. Hydrated media for the SUF process were sterile-filtered through a 0.22-µm filter; for the multiuse fermentor, they were sterilized in situ. After initial preparation, we added sterile tetracycline and chloramphenicol (MilliporeSigma) at final concentrations of 10 and 50 µg/mL, respectively, to the media before inoculating the fermentors.
Cultivation: Seed Culture Growth: A 500-mL culture grew overnight in a 2.5-L sterile Thompson disposable polycarbonate Erlenmeyer flask to an OD600 range of 12.0 ± 4.0. After storage at 4–8 °C, that culture was used to inoculate the bioreactor for batch cultivation within 24 hours of preparation.

Figure 1: Fermentor setups; (LEFT) Applikon 20-L stainless-steel steam-in-place fermentor (https://www.applikon-biotechnology.com); (RIGHT) Thermo Fisher Scientific HyPerforma S.U.F. 30 single-use fermentor (https://www.thermofisher.com) with
Applikon eZ-Control process control system.
Fermentation in Stainless Steel: Batch culture in the Applikon SIP fermentor was carried out with a working volume of 10 L (Figure 1a) and a system aspect ratio of 3:1. This fermentor has a top drive, and the shaft is equipped with three six-blade Rushton-type disk impellers. The vessel is mounted with four baffles to minimize the influence of eddy currents from stirring and equipped with an Applikon eZ-Control controller. In addition to standard sensors for pH, DO, temperature, and pressure, the system also monitors O2 and CO2 in the exhaust gas through a BlueInOne Ferm off-gas sensor (BlueSens).
Before the start of fermentation, we calibrated the DO sensor to 100% under these settings: 200-mbar pressure, 10-SLPM air flow, 800-rpm stirring, and 37 °C temperature. Fermentation began at a temperature of 37 °C, a pressure of 200-mbar, an air-flow rate of 1.0 vvm, and pH controlled at 6.8, with a dead zone of 0.1. We used the seed culture (grown to exponential phase) to inoculate this fermentor to OD600 at 0.7–1.0 and a start volume of 8.0 L. Feeding started when OD600 reached >1.0, usually within three hours after inoculation. The feeding rate was constant at 3.0 mL/L/h. To induce rFab expression, we added IPTG and arabinose when the OD600 reached the target of ~50.0 ± 4.0. After induction, fermentation continued for another 12–15 hours, and then we harvested when the OD600 reached a target of about 100.0 ± 10.0. The setpoint of DO was ≥30%, and the cascade enrichment of pure oxygen flow rate was set to ≤5 SLPM.
SUF Fermentation: The Thermo Scientific HyPerforma 30 S.U.F. system comes standard with a sterile single-use vessel for mixing, exhaust ventilation, and air sparging with oxygen-enrichment options (Figure 1b). It uses an Applikon eZ-Control system with modules for controlling or monitoring of agitation, air flow, temperature, DO, and pH. Ports are available to accommodate aseptic connections for addition, sampling, and harvest. Detailed descriptions of these functions and system specifications can be found on the supplier’s website (11).
Before inoculation of this fermentor, we calibrated the DO sensor to 100% under these settings: 30-SLPM or 1.0-vvm air flow, 524-rpm stirring, and 37 °C temperature. Fermentation began at a temperature of 37 °C, air-flow rate of 30.0 SLPM, and pH controlled at 6.8, with a dead zone of 0.1. We used the seed culture grown to exponential phase to inoculate this fermentor to OD600 at 0.7–1.0 at a starting volume of 24.0 L. Feeding started when OD600 reached >1.0, usually within three hours after inoculation. The feeding rate was constant at 3.0 mL/L/h. To induce rFab expression, we added IPTG and arabinose when the OD600 reached the target of ~50.0 ± 4.0. After induction, fermentation continued for another 12–15 hours. We harvested when the OD600 reached a target of ~100.0 ± 10.0. The harvest volume was about 30 L, approaching the upper limit of the fermentor’s capacity.
It is well known that disposable bioprocess containers (BPCs) cannot withstand higher backpressures. Therefore, the SUF has no pressure setpoint (unlike the SIP fermentor), and the actual pressure was attributable to air flow/oxygen enrichment and slow egress from the exhaust filter. The DO setpoint was ≥30%, and the cascade enrichment of pure oxygen flow was ≤30 SLPM (1.0 vvm). As a safety feature, the system has internal controls to keep backpressures from exceeding 0.5 psi. The controller compares pressure measured by a PendoTech in-line sensor against the limit value, and a safety lock actuates mass-flow controllers to modify air-flow and oxygen-enrichment rates to limit pressure.
Purification: An equivalent portion of cell paste from each fermentation was suspended in 2 L of lysis buffer and lysed by continuous sonication for an hour at 80 amps. After centrifugation and filtration, soluble rFab was purified using cOmplete His-tag resin (Roche) followed by HyTrap Q HP anion-exchange chromatography (Cytiva) in flow-through mode. Diafiltration placed the purified rFab into a final storage buffer. The details of purification and final formulation are proprietary to our company.
Analytical Method: During the fermentation, we collected 10-mL samples at multiple time points. The optical density of these samples was measured at 600 nm by a Shimadzu UV160U spectrophotometer. For harvesting, we operated a Beckman Coulter J2-21 centrifuge with a JLA-8.1 rotor at 13,000 rpm for 25 minutes. After decanting the supernatant, we calculated the weight of the cell pellet as the wet weight of biomass growth and purified the cell paste. The resulting rFab was evaluated by absorption at 280 nm, size-exclusion high-performance liquid chromatography (SEC-HPLC), mass spectrometry, and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), which showed that the purity was >98% (data not shown).
Results
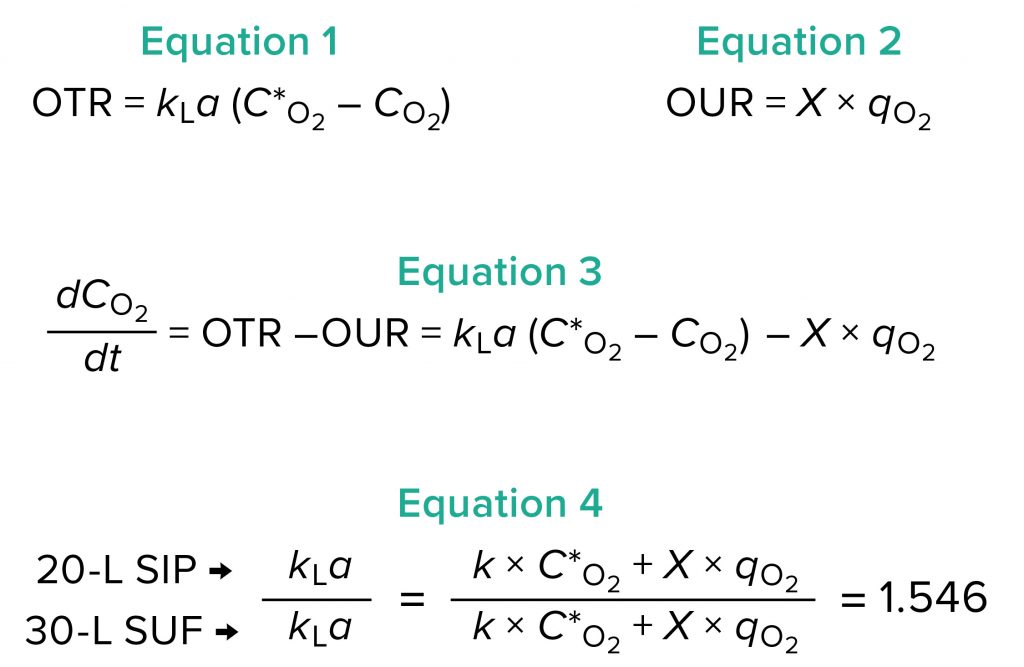
Expression of rFab in SIP Fermentor at 10-L Scale: For fermentation development based on data shown in Figure 2a, the Applikon ez-Control 20-L fermentor (Figure 1a) was equipped with a pure-oxygen enrichment module. The fermentor was inoculated with a 0.45-L seed from shaker flasks with growth to OD600 of 12.84, which was in the exponential phase. To control DO ≥30% (setpoint), the cascade scheme activated oxygen enrichment flow rate only ≤5 SLPM. That simple strategy proved to be effective for this process.
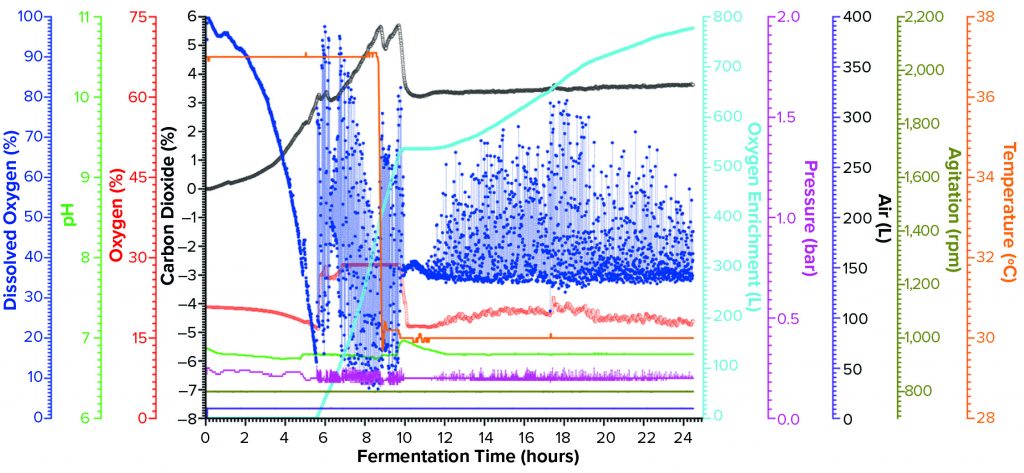
Figure 2a: Fermentation profile of the Applikon eZ-Control 20-L system; pure oxygen supplementation was initiated at the 5:17 time point. Temperature shift (from 37 °C to 30 °C) was initiated at induction (8.5 hours of growth). The right-side y-axes show fermentation parameters that could be controlled actively during growth (O2 enrichment, pressure, air flow, agitation, and temperature). The left-side y-axes show parameters that were monitored during growth (dissolved oxygen, pH, and concentrations of O2 and CO2).
Protein expression was induced by adding IPTG to a concentration of 1.0 mM and arabinose to 12.3 mM at 8.53 hours, at which time the OD600 value was measured at 49.0. During induction, the temperature was reduced from 37 °C to 30 °C and then held constant for the rest of the run. Feeding began at a constant flow rate of 3.0 mL/L/h as early as 58 minutes after inoculation, with a measured OD600 of 1.15. We kept the feeding rate constant throughout the process, adding a total of 711 mL by the end. Harvesting this fermentation 15.8 hours after induction (at an OD600 of 98.8) provided 1,127.3 g of cell paste. Purification yielded rFab at 0.976 mg/g of cell paste.
Expression of rFab in a Single-Use Fermentor at 30-L Scale: For this fermentation (Figure 2b), we used a 1.4-L seed from three Erlenmeyer flasks after overnight growth, with an OD600 of 12.4 to inoculate the fermentor at an initial working volume of 24.0 L. During the growth process, the system actively controlled air-flow rate, stirring, pH, and temperature parameters and monitored DO, enrichment-oxygen flow rate, pressure, and accumulated consumption of both air and oxygen.

Figure 2b: Fermentation profile of the Thermo Fisher Scientific HyPerforma S.U.F. 30 single-use fermentor; the right y-axes show fermentation parameters that could be controlled actively during growth (pressure, airflow, agitation, and temperature); the left y-axes show parameters that were monitored during growth (DO, pH, and acid/alkaline consumptions).
We set the target DO value at ≥30%, with pure-oxygen flow cascade for enrichment ≤15 SLPM. However, when the actual demand for oxygen enrichment began (as early as 2.76 hours after inoculation), we found that oxygen flow rate did not control DO sufficiently to the set value. First, we increased the upper limit of the pure-oxygen flow rate from 15 SLPM to 30 SLPM while maintaining the air flow rate at 30 SLPM. But soon we discovered that the oxygen flow rate could not exceed 20 SLPM (when the DO dropped to nearly 0%) because of the system-safety interlock that limits backpressure from exceeding 0.5 bar. Consequently, at hour 6.43 we reduced the air-flow rate from 30 to 15 SLPM to allow the enrichment-oxygen flow rate to reach 30 SLPM. That strategy for DO control remained in place for the rest of the process.
Feeding at a constant rate of 3.0 mL/L/h began 1.85 hours after inoculation, when OD600 was measured at 2.38. We kept the feeding speed constant throughout this process, adding nearly 2 L in total by the end. Fermentation was induced through addition of IPTG and arabinose solutions to final concentrations of 1.0 mM and 12.3 mM, respectively, at 8.42 hours and an OD600 of 44.9. At that time, temperature was reduced from 37 °C to 30 °C while all other settings remained unchanged. We harvested this fermentation after 25 hours of growth to an OD600 of 112.4, obtaining 3,660.3 g of cell paste. Purifying part of that cell paste yielded 1.012 mg of rFab per gram of paste.
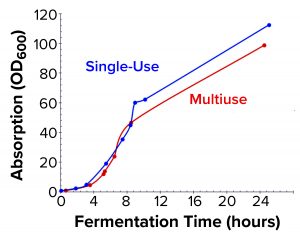
Figure 3a: Biomass growth curves from the two fermentations are compared based on OD600 measurements.
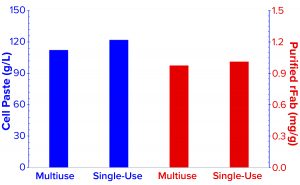
Figure 3b: Cell paste and purified rFab yields are compared for the two fermentations. For direct comparison, the cell paste amount was normalized to yield out of 1-L fermentation broth at harvest, and a purified-rFab number was calculated to yield from each gram cell paste harvest.
Comparison of E. coli Biomass Growth and rFab Yields: Figure 3a shows a typical E. coli biomass growth curve from the fermentation used to produce rFab in the two fermentors. We measured OD600 only during normal working hours in the early stages to monitor for induction time. E. coli growth was nearly the same in the two fermentor platforms. Whole broth from each fermentor was harvested at the end of the process through a single centrifugation step to collect cell paste. Wet biomass yields from the Applikon SIP fermentor and the Thermo Scientific HyPerforma 30 S.U.F. were 112.3 g/L and 122.01 g/L of fermentation broth, respectively (Figure 3b). We processed the entire cell paste from the former through one purification batch and an equivalent partial paste from the latter using the same steps. Yields were comparable: 0.976 mg and 1.012 mg of rFab per gram of cell paste, respectively.
Oxygen Enrichment Consumption to Maintain Dissolved Oxygen Control: Before inoculation, both fermentors were calibrated to 100% DO. Then we added freshly grown inoculum to an OD600 of 0.7 to start batch growth. The original DO control strategy was the same, with a set point of 30%, and cascaded with oxygen enrichment at a flow rate up to 50% of the air flow rate. In the SIP fermentor, demand for oxygen enrichment began at 5.23 hours when DO fell below 30% for the first time. For most of the fermentation time, the system controlled DO at setpoint, ultimately using a total of 777.2 L oxygen. Thus, one full 64-lb K-style cylinder (Praxair 249CF) of compressed oxygen would be sufficient for this setup to run 9–10 similar fermentation batches. We calculated the average oxygen consumption per liter of fermentation volume to be about 4.0 L/h (Figure 5).
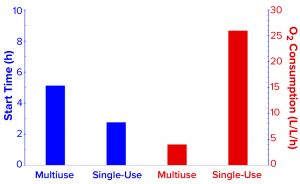
Figure 4: Comparing the start of demand for oxygen enrichment and oxygen-enrichment consumption rates of the two fermentors.

Figure 5: Comparing partial profiles of dissolved oxygen (DO) showing the start of demand for pure oxygen enrichment; the slope for initial dropping of DO from each curve was fitted with first-order kinetics (equation and R2 shown). The time when DO first dropped to a setpoint of 30% for cascade control also is shown.
In the SUF system, DO dropped <30% as early as 2.8 hours after the start of fermentation, thus requiring oxygen enrichment much sooner (Figures 4 and 5). After our many efforts to improve DO control, the results and profile remained poor. We had installed two full cylinders of compressed oxygen at the start, expecting to use ~14,000 L for the batch. But they lasted only 16.2 hours, leaving no oxygen enrichment available for 6.7 hours while the process still needed it. We calculated the average oxygen consumption per liter of fermentation volume to be ~26.0 L/h (Figure 5), which was 5.5Ă— higher than that of the multiuse-fermentor system.
Discussion
Characterization of Oxygen Transfer: Many microbial fermentations are aerobic processes with high demand for oxygen. Because its solubility in fermentation medium is very low, ensuring sufficient oxygen for microorganisms is difficult. Thus, characterizing the oxygen transfer rate (OTR) is one of the most important parameters in evaluating both conventional SIP and single-use fermentors. OTR can be determined based on Equation 1.
During fermentation evaluation and monitoring, we record many on-line and off-line parameters: optical density at 600Â nm, dry and wet cell weights (DCW, WCW), DO, pH, temperature, and concentrations of oxygen and carbon dioxide in the exhaust gas, as well as other metabolites. The oxygen uptake rate (OUR) is calculated from those parameters using Equation 2.
Based on the calculated OTR and OUR, Equation 3 provides the oxygen transfer balance for the liquid phase. This equation contains the important volumetric mass transfer coefficient (kLa), which is widely accepted for evaluating the effectiveness of oxygen mass transfer in both SUF and SIP fermentors — and for comparing different systems (12). The kLa value depends on equipment-specific and process-operation parameters in addition to media properties.
Some standard methods can be used to determine kLa experimentally. During cultivation, it can be calculated from a recorded DO curve. From the on-line DO profile, the initial drop slope (k) can be fitted with first-order kinetics (Figure 5). That slope provides important information on volumetric mass transfer. Note that the margin of error to calculate kLa from a DO curve directly will be higher because of the difficulty in determining an off-line biomass value at small intervals. But to compare different systems, we need only to determine kLa ratios between them rather than calculate the exact value for each system. With some mathematical manipulation, we can obtain Equation 4 based on Equations 1–3. Thus, we calculated the ratio of kLa for the Applikon SIP fermentor to that of the Thermo Scientific HyPerforma 30 S.U.F. system to be 1.546.
Reasons for the Difference in Oxygen Transfer: Many factors related to equipment design and operating parameters could be affecting the SUF’s oxygen-transfer performance in this case. Equipment-specific parameters include the vessel’s ratio of height to diameter, the impeller type and geometry, number of baffles, and so on. Although the HyPerforma S.U.F. platform design attempts to mimic the design principles of a traditional SIP fermentor, disposable bioprocess container (BPC) materials and aseptic-processing requirements limit its structure. The same design challenges also limit the system’s operating range of certain parameters. Fermentation in the SUF was carried out with stirring at 524 rpm, which is the equipment’s upper limit; fermentation in the multiuse system used a stirring speed of 800 rpm, with room to increase that further to a maximum limit of 1,500 rpm.
Another well-known operating parameter limited in SUFs is backpressure. Pressurization of SIP fermentors has proven to be a valuable and scalable tool for OTR enhancement in microbial fermentation. At elevated exhaust pressures, oxygen limitations can be circumvented in many high–cell-density processes (13). Stainless-steel vessels and jackets for many fermentors can meet pressure requirements up to 3.0 bar. Although safety and energy cost considerations prevent fermentation from being carried out under such high pressure, such processes often run at a pressure of 0.75–1.0 bar. By comparison, fermentation in the Applikon SIP fermentor was carried out at 200 mbar, which was far below the normal range. It is conceivable that if that fermentation were carried out within the upper limit of stirring and pressure, the gap in oxygen transfer would be even greater between the SUF and multiuse fermentors.
To alleviate the restrictions of agitation and pressure on oxygen transfer, SUF systems rely on pure oxygen. From the oxygen-consumption rate we observed, it seems necessary for optimal control of DO to replace air flow with oxygen flow rather than enriching air with oxygen. Supplying oxygen with conventional compressed-oxygen cylinders is unlikely to meet those requirements. Although we tested a “high-density” process with OD600 of about 100, many published reports show growth of E. coli in SIP fermentors to densities >300. We find that it would be more difficult to reach such high densities in the Thermo Scientific HyPerforma 30 S.U.F. even with proper DO control.
Fermentation in single-use system was comparable to that in a multiuse fermentor in terms of growth profile, biomass, and purified rFab yield. The advantages of single-use technology remain, including convenience and flexibility as an alternative to the SIP platform. But our results, though acceptable, demonstrate a weakness in oxygen transfer with single-use fermentors.
References
1 Eibl R, Eibl D. Single-Use Technology in Biopharmaceutical Manufacture. John Wiley and Sons: Hoboken, NJ, 2019.
2 29-0564-39 AB. Microbial Fermentation in Single-Use Xcellerex XDR-50 MO Fermentor System. GE Healthcare: Uppsala, Sweden, February 2014; https://cdn.gelifesciences.com/dmm3bwsv3/AssetStream.aspx?mediaformatid=10061&destinationid=10016&assetid=17083.
3 Brown J, et al. CO29180: Scale-Up of Microbial Fermentation Using Recombinant E. coli in HyPerforma 30 L and 300 L Single-Use Fermentors. Thermo Fisher Scientific: San Jose, CA, 2014; https://www.thermofisher.com/content/dam/LifeTech/Documents/PDFs/CO29180-SUF-Launch-AppNotes-Scale-Up%20of%20Microbial-Global-FLR_V2.pdf.
4 Ding Y, Zeck B, and Allen SP. Comparative Study of Single-Use and Reusable Fermentors: Production of Recombinant Proteins Through Bacterial Fermentation. BioProcess Int. 17(10) 2019: 24–31; https://lne.e92.mwp.accessdomain.com/upstream-processing/upstream-single-use-technologies/comparative-study-of-single-use-and-reusable-fermentors-production-of-recombinant-proteins-through-bacterial-fermentation.
5 Celebrating the Best BPI Contributions on Cell Culture and Fermentation. BioProcess Int. 18(11) 2020: e1; https://bpi.bioprocessintl.com/2020-readers-choice-awards-upstream.
6 Shuler ML, Kargi F. Bioprocess Engineering: Basic Concepts. Prentice Hall: Englewood Cliffs, NJ, 1992.
7 Ukkonen K, et al. Effect of Culture Medium, Host Strain and Oxygen Transfer on Recombinant Fab Antibody Fragment Yield and Leakage to Medium in Shaken E. coli Cultures. Microb. Cell Fact. 12, 2013: 73; https://doi.org/10.1186/1475-2859-12-73.
8 Schein CH. Production of Soluble Recombinant Proteins in Bacteria. BioTechnol. 7, 1989: 1141–1148; https://doi.org/10.1038/nbt1189-1141.
9 Pack P, et al. Improved Bivalent Miniantibodies, with Identical Avidity As Whole Antibodies, Produced By High Cell Density Fermentation of Escherichia coli. Nat. Biotechnol. 11(11) 1993: 1271–1277; https://www.nature.com/articles/nbt1193-1271.
10 Ding Y, et al. Oxygen Control Strategy and Yield of Recombinant Antibody Fragments Produced in Fermentation. BioProcess Int. 19 (1–2) 2021: 38–41; https://lne.e92.mwp.accessdomain.com/upstream-processing/fermentation/rfab-yield-oxygen-control-strategy-for-antibody-fragments-produced-in-fermentation.
11 Catalog No. SUF0030.9001. HyPerforma Single-Use Fermentor Systems, 30L, Jacketed, AC Motor, with 2 position vent filter bracket. Thermo Fisher Scientific: San Jose, CA; https://www.thermofisher.com/order/catalog/product/SUF0030.9001#/SUF0030.9001.
12 Meusel W, et al. Recommendations for Process Engineering Characterization of Single-Use Bioreactors and Mixing Systems By Using Experimental Methods. DECHEMA Biotechnologie: Frankfurt am Main, Germany, February 2016; https://dechema.de/dechema_media/Downloads/Positionspapiere/Single_Use_BioReactors_2020-p-20006899.pdf.
13 Knoll A, et al. High Cell Density Cultivation of Recombinant Yeasts and Bacteria Under Non-Pressurized and Pressurized Conditions in Stirred Tank Bioreactors. J. Biotechnol. 132(2) 2007: 167–179; https://doi.org/10.1016/j.jbiotec.2007.06.010.
Corresponding author Yongxue Ding is principal scientist, You Pan is principal research scientist, Mark Gibson is group leader of the propagation team, Troy McSherry is leader of the purification group, and Steven P. Allen is a Volwiler research fellow and manager of biologics process design and analytical chemistry research and development, all at Abbott Diagnostics Division, Department 09NC, AP8A, Abbott Laboratories, 100 Abbott Park Road, Abbott Park, IL 60064; 1-224-668-3629; yongxue.ding@abbott.com.
| Antibody Fragments Are Increasing Demand for Microbial Capacity by Dan Stanton
As it expands capacity in Switzerland, Lonza Pharma & Biotech says that demand for antibody-fragment, scaffold, and bioconjugate services is driving microbial manufacturing (1). The contract development and manufacturing organization (CDMO) will add a sixth microbial manufacturing suite — currently under construction at Lonza’s biopark in Visp, Switzerland. The 3,000-L of additional microbial capacity will serve an extended manufacturing agreement with French drug developer Servier Laboratories to produce an asparaginase-based active pharmaceutical ingredient (API) for therapies to treat acute lymphoblastic leukemia (ALL). According to Lonza representatives, the additional plant represents an increased demand for microbial biomanufacturing services that the CDMO is seeing across the industry. Clemens Jakobi (Lonza’s commercial director) said, “We have observed a very strong demand increase for manufacturing solutions for antibody fragments, scaffolds, and nanobodies, but also bioconjugates over [recent] years. And this trend continues, driving microbial fermentation fast forward [because] bacteria and yeasts can easily express smaller biomolecules. On top of this, drug-substance development in microbial is usually faster, and microbial processes can be optimized for yield and scale.” Jakobi added that the recent push for vaccine development arising from the COVID-19 crisis has brought more attention to microbially based biomanufacturing. “The need for vaccine manufacturing capacity, irrespective of the underlying manufacturing technology, has increased. mRNA manufacturing requires pDNA as a key raw material, which also originates from microbial manufacturing. And we are proud that Lonza masters both technologies.” Axel Erler is the company’s associate director of commercial development for microbial biopharmaceuticals. He said that although microbial fermentation can handle a much larger degree of molecular diversity than ever before, some difficulties remain. “The necessity of molecule-tailored manufacturing processes requires an in-depth know-how and, associated with that, capacity for development services. And of course, we are expanding our services in sync with the expansion of our manufacturing capacities.” Erler added that the team in Visp has been involved for over 30 years in microbial fermentation — and the staff has been growing steadily. “Today, our end-to-end offering ranges from an extensive microbial expression toolbox for clinical supply up to profound experience in the market launch of new products and subsequent commercial production.” Lonza currently offers small-scale (70 L and 1,000 L) and large-scale (15,000 L) microbial fermentation capacity, Jakobi said. “Given that the demand is quite strong, our ambition is to use as much capacity as possible to provide patients with access to life-saving therapies. That is why we are now expanding our capacities to extend our service offering in microbial.” The new facility is expected to be operational in the second half of 2022, and Lonza expects to add 100 employees to the existing microbial team. Reference Based in Montpellier, France, Dan Stanton is founding editor of BioProcess Insider; 44-7552-290-774; dan.stanton@informa.com. |