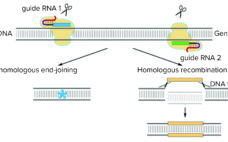
Since January 2016 (with a brief interlude as described below), the Patent Trial and Appeal Board has been attempting to adjudicate the proper inventorship of CRISPR technology in (to date) six separate patent-interference proceedings. (Scientific “priority has been decided, for now, by the awarding of the Nobel Prize in Chemistry to Jennifer Doudna and Emmanuelle Charpentier in 2020; see the “Priority Claims” box). CRISPR-based gene editing was hailed as the “Breakthrough of the Year” in 2015 (1), and the scientific…
Author Archives: Kevin E. Noonan
The Biosimilars Action Plan: Promoting Faster and More Extensive Adoption of Biosimilar Drugs
The pace with which biosimilar drugs have been adopted in the United States has frustrated (and displeased) policymakers (1). After passage of the Biologics Price Competition and Innovation Act (BPCIA) (2) as part of the Affordable Care Act of 2010 (3), policymakers intended and expected significant reductions in expenditures for this class of biopharmaceuticals (4). The Federal Trade Commission (FTC) had predicted that the percentage of savings would be lower than that of the <90% reduction in costs for small-molecule…
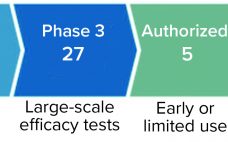
Intellectual Property and COVID-19
The COVID-19 pandemic is the greatest global health threat caused by a virus since the influenza pandemic of 1918 (and, before that, innumerable smallpox outbreaks throughout history). So far, the number of infections and deaths has not reached levels seen during the “Spanish flu” pandemic. However, travel, global trade, and modern factors such as misinformation on social media have increased infection rates and risks of infection. As of 15 July 2021, the World Health Organization (WHO) had reported 188,655,968 confirmed…