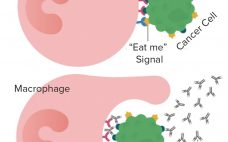
Forty Seven is a company developing novel therapies based on anti-CD47 and other immuno-oncologies. CD47 is called the “don’t eat me” signal that cancer cells give out to escape elimination by the body’s immune system. Qinghai Zhao, vice president of technical development and manufacturing, is one of the scientists working on the company’s magrolimab (5F9) monoclonal antibody that is designed to block the binding of the CD47 signal to the cell receptor SIRP-α while boosting the “eat me” signal that…
Upstream Development
Creating Novel Cell Lines By Genome Editing: Simplifying Cell-Based Assays and Improving Production of Biomolecules
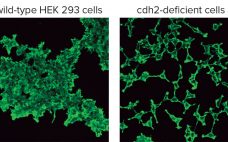
Cultured cell lines have a diverse range of applications. They are used broadly by cell biologists, clinicians, tissue engineers, biotechnology scientists, and bioengineers. The most important uses of cell culture are in the cell-based assays and production of biologically active recombinant proteins. In recent years, genome editing has been used widely to study the structure, function, and localization of endogenous proteins in cultured cells. However, applying the same genome editing techniques to cell lines also could improve the propagation of…
Streamlined Serum-Free Adaptation of CHO-DG44 Cells: Using a Novel Chemically Defined Medium
Monoclonal antibodies (MAbs) have radically transformed the treatment of many chronic diseases, mainly in the fields of oncology and autoimmunity. The overwhelming majority of therapeutic MAbs are manufactured from recombinant Chinese hamster ovary (CHO) cell lines. The original CHO cell line was isolated in the 1950s, and since the early 1980s, it has become the workhorse of the biopharmaceutical industry. The CHO-DG44 strain was generated after several rounds of mutagenesis that deleted both copies of dihydrofolate reductase (dhfr) genes by…
Monoclonal Antibodies: Beyond the Platform in Manufacturing
The vast majority of monoclonal antibody (MAb) production processes are based on fed-batch Chinese hamster ovary (CHO) cell culture and protein A affinity column chromatography capture. Increasing cost-consciousness — among innovator companies as well as biosimilar makers — has many companies looking “beyond the platform” for less expensive alternatives that may provide better results. Here the BPI editors review some state-of-the-art alternatives in upstream and downstream MAb drug substance bioprocessing as well as drug-product manufacturing. The current “gold standard” platform…
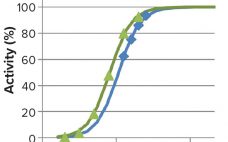
Apparent Matrix Effects in an Iduronate 2-Sulfatase Specific Activity Assay
The recombinant fusion protein SHP631 consists of a chimeric monoclonal antibody binding to human insulin receptor and iduronate-2-sulfatase (I2S). This product is being developed as an enzyme replacement therapy to treat cognitive symptoms of Hunter’s syndrome. Because the current therapy (idursulfase, brand name Elaprase from Shire) cannot cross the blood–brain barrier (BBB), SHP631 is being developed to do so, enabling the presence of I2S in the brain. The enzymatic activity of this molecule is measured using the substrate 4-methyl umbelliferyl-α-L-idopyranosiduronic…
Aspects of Acceleration: Biomanufacturers Need Smart Strategies to Speed Products to Market
No matter what the industry, it’s widely accepted that slow-moving companies give their nimbler competitors an advantage, allowing them room to dominate the market even if their products are not superior. “Me-too” products and their sponsors often are seen as followers rather than leaders — even if they offer improvements over what is already available. Fast movers are flexible and adaptive to a dynamic business environment. They capitalize on opportunities and navigate risks and challenges by responding quickly to changes…
Cell Culture Scale-Up in Stirred-Tank Single-Use Bioreactors
Bioprocess development usually is carried out in systems with small working volumes. This helps save time and resources because, at small scale, several experiments can be conducted in parallel. Costs for media are kept low, and relatively little laboratory space is required to operate small-scale bioreactors. But over the course of development, biopharmaceutical companies need more material for characterization, trial runs, and finally for commercialization. They transition to bench scale and then up to pilot or production scale with the…
Accelerating Biopharmaceutical Development with High-Throughput Glycan Screening and Multiple Attribute Methodology
Part 1 Development of biopharmaceuticals comprises many integrated steps, beginning with research and discovery and optimally ending with a commercial therapeutic molecule. Early screening of large numbers of clones and cell culture expression conditions is essential to identifying proteins that carry to greatest likelihood of clinical and commercial success. Part one of this report reviews how high-throughput glycan screening can significantly improve current analytical strategies relating to cell line development. Part 2 Minor impurities and changes in attributes such as…
Assurance of Clonality: Next-Generation Single-Cell Dispensing in Cell Line Development and Single-Cell Genomics
At the Cell Line Development and Engineering (CLD&E) conference(23–25 April 2018, Amsterdam), Jonas Schöndube, CEO of cytena GmbH, gave a presentation highlighting some of the company’s recent developments in single-cell dispensing for documented clonal cell lines. About cytena cytena is a young company dedicated to the development and manufacture of tools for the biopharmaceutical industry. In 2015, cytena launched the single-cell printer™ (scp™), which enables fully automated isolation of single cells into 96- and 384-well plates. The patented technology uses…
eBook: Quality By Design for Monoclonal Antibodies — Establishing the Foundations for Process Development, Design Space, and Process Control Strategies
The quality by design (QbD) modernized approach to pharmaceutical development is intended to provide regulatory flexibility, increased development and manufacturing efficiency, and greater room to innovate as well as improve manufacturing processes within defined ranges without obtaining regulatory approval first. QbD is a systematic developmental approach that starts with a clear goal in mind and emphasizes understanding of how variability in both process and materials affects a final product (1). Historically, product quality has been assured either with end-product testing…