Ray Price (senior director of business development, DiscoveRx) 3:30–3:55 pm Advances in Research Tools to Accelerate Drug Development Price introduced the BioSeek drug-discovery platform with examples. The technology is built on three pillars: primary human cells; models that use growth factors or cytokines to model a disease environment and then predict how drugs change biomarker responses in those systems; and comparisons of generated profiles with a reference database of more than 4,000 compounds. DiscoveRx uses that database and informatics tools…
Upstream Development
Challenges in Implementing Quality By Design: An Industry Perspective
In the fall of 2004, the US Food and Drug Administration (FDA) published a final report entitled Pharmaceutical CGMPs for the 21st Century: A Risk-Based Approach (1). This publication set the groundwork for a prospective risk‑based approach to pharmaceutical product development. It was published on the heels of a November 2003 agreement between the FDA and the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) to develop an internationally harmonized plan for developing…
Bioreactor Design for Adherent Cell Culture — The Bolt-On Bioreactor Project, Part 3: Containment, Sterility
The Bolt-on Bioreactor (BoB) project is an independent initiative aimed at developing and commercializing a bioreactor for the automated and efficient culture of adherent cells, especially for application in the production of therapeutic cells and other biopharmaceuticals (1). After conducting thorough research on available culture systems for adherent cells, the BoB team believes that a successful alternative to existing devices must answer four major challenges. Addressed in the first article of this series (2), the first challenge has to do…
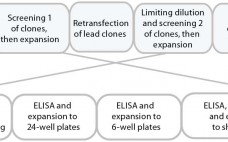
Rapid Development and Scale-Up of Biosimilar Trastuzumab: A Case Study of Integrated Cell Line and Process Development
Compared with that for new drugs, biosimilar development faces significantly condensed timelines from cell line to first-in-human (FIH) trials. A biosimilar development program needs to accelerate quickly toward preclinical and phase 1 studies; phase 2 studies typically are not required because dose response and other patient-treatment concepts are already established by the original, comparator medicine. Phase 3 studies typically are limited to fewer patients, which ultimately shortens overall timelines and costs. The key challenge remains: demonstrating comparability and high similarity…
Bioreactor Design for Adherent Cell Culture: The Bolt-On Bioreactor Project, Part 2 — Process Automation
The Bolt-on Bioreactor (BoB) project is an independent initiative to develop and commercialize a bioreactor for automated and efficient culture of adherent cells, especially in production of therapeutic cells and other biopharmaceuticals (1). After conducting thorough research on available culture systems for adherent cells, the BoB team believes that a successful alternative to existing devices must solve four major challenges. Addressed in the first installment of this series (2), the first challenge concerns volumetric productivity. The second challenge is…
A Multidisciplinary Approach to Manufacturing Biotherapeutics
Optimizing antibody manufacturing processes has gone beyond the first-order goal of achieving elevated protein titers and now also focuses on understanding biologic and manufacturing process variables that define cellular machinery and protein quality. A holistic approach to biotherapeutic manufacturing incorporates several applied disciplines such as biology, engineering, process control, signal processing, and modeling to reduce the “black-box” model of cell- based protein production into an operational design space. This is in line with the US Food and Drug Administration’s quality…
30 Years of Upstream Productivity Improvements
We recently completed an analysis of the past 30 years of industry progress in commercial-scale expression titers and bioprocessing yields. These basic measures of biopharmaceutical manufacturing efficiency also benchmark the technological progress made in bioprocessing over recent decades. Titer and yield improvements generally indicate related bioprocessing cost savings, something most commercial-scale manufacturers work to improve. This focus on efficiency and productivity has led to constant bioprocessing improvements even for long-approved and -marketed products. Our findings indicate that although upstream titers…
Bioreactor Design for Adherent Cell Culture — The Bolt-On Bioreactor Project, Part 1: Volumetric Productivity
The Bolt-on Bioreactor (BoB) project is an independent initiative aimed at developing and commercializing a bioreactor for efficient, automated culture of adherent cells in production of therapeutic cells and other biopharmaceuticals (1). After conducting thorough research on available culture systems for adherent cells, the BoB team believes that a successful alternative to existing devices must solve four major challenges. The first challenge has to do with volumetric productivity, the second with process automation, the third with containment and sterility, and…
Accelerated Product Development: Leveraging Industry and Regulator Knowledge to Bring Products to Patients Quickly
A Chemistry, Manufacturing and Controls (CMC) Strategy Forum titled “Accelerated Product Development: Leveraging Combined Industry and Regulator Knowledge to Bring Products to Patients More Quickly” was held in Washington, DC, on 27 January 2014. Biological therapeutics in development are demonstrating remarkable results in the clinic for many indications. So companies are seeking ways to accelerate the approval of these therapies and rapidly bring them to market. Many such products take the form of well-characterized proteins (e.g., IgG1 or IgG2 monoclonal…
Ask the Expert: Accelerating Bioprocess Development Using Shake-Flask Metabolic Activity Data
with Dr. Gernot John of PreSens Precision Sensing GmbH Shake flasks are simple, low-cost devices widely used in screening and media optimization. The most widely measured parameter to determine biomass is optical density (OD). It is typically measured offline because no suitable equipment for broad range biomass measurements in shake flasks has been available. But a new, compact, SFR vario device from PreSens Precision Sensing can be placed under shake flasks to measure four parameters online: pH, O2 saturation, oxygen…