Bioassay development is foundational to the well-characterized biotechnology product paradigm. Bioassays are the best tools for drug developers to use in determining the biological activity (potency) of their products, which has been a biopharmaceutical critical quality attribute (CQA) since long before that concept had a name. Thus, these assays are vital to quality assurance and quality control (QA/QC), preclinical studies, and clinical testing — and by extension to process development and monitoring. Because of their complex nature, bioassays are among…
QA/QC
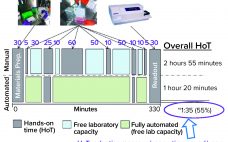
Automation of Potency Assays: A Strategic Journey
Cell-based potency testing provides quantitative data concerning a drug’s biological activity. Thus, it plays an essential role in biopharmaceutical quality control (QC), good manufacturing practice (GMP) product release, comparability determination, and stability testing for both drug products and drug substances. Potency is a critical quality attribute (CQA) often scrutinized by regulators and reviewers. Test methods are specific to a drug’s mechanism of action (MoA) and should be validated to internationally harmonized regulatory standards (1). The options preclude applying a simple…
HCP Assay Development: Managing Risks with Evolving Technologies
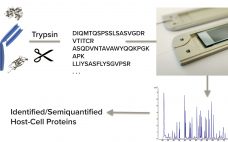
Host-cell proteins (HCPs) are major impurities of concern in biomanufacturing. When present in drug formulations, they can reduce efficacy (by compromising product stability), introduce toxicity, and increase a recipient’s risk for long-term immunogenicity. Understanding HCP profiles and integrating effective removal strategies are important parts of developing new biological drugs — to fulfill regulatory guidelines and to ensure patient safety through product quality. HCP populations can be both complex and structurally diverse, and some changes in upstream culture conditions can affect…
Stability Testing: Monitoring Biological Product Quality Over Time
Many physical and chemical factors can affect the quality, safety, and efficacy of biopharmaceutical products, particularly after long-term storage in a container–closure system that can be subject to variations in temperature and light, as well as agitation with shipping and handling. Proteins are inherently complex physiochemically, from their primary amino acid sequences to their higher-order structures, and they require specific conditions to maintain their integrity and functionality. Advanced biological therapies can be even more complicated and particular about their environments.…
Emerging Strategies for Drug Product Comparability and Process Validation: Part 1 — Analytical Tools and Drug Product Comparability
Process validation is a key part of the development and manufacture of all approved drug products, but its completion can be a daunting task. At a two-day CASSS CMC Strategy Forum held in July 2016 in Gaithersburg, MD, speakers and attendees addressed the many technical, practical, and regulatory facets of drug product process validation and comparability. In part 1 of this report, we summarize the key discussion points of the first day, which focused on analytics and comparability. Session One:…
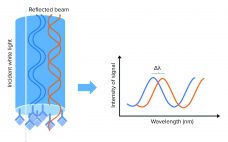
Customized Yeast HCP Quantification with Biolayer Interferometry Using a Horseradish Peroxidase Substrate
Biopharmaceuticals are the largest group of drugs under development (1), and the demand for new and safe drug products is high. The most common bacterial and mammalian cell lines for production are Escherichia coli, Chinese hamster ovary (CHO) cells, and yeast. During a production bioprocess, a cell line expresses not only the molecule of interest, but also host-cell proteins (HCPs). They are considered to be impurities in a final drug product because they can affect the efficacy and safety of…
Ask the Expert: Developing Strategic Analytical Programs for Therapeutic Peptides
Ashleigh Wake began her 15 October 2020 “Ask the Expert” presentation by pointing out that peptide products are manufactured in a “regulatory vacuum.” Peptide-product developers must be strategic in designing characterization and quality control (QC) programs. Wake reviewed available methods and explored key considerations for developing phase-appropriate analytical controls. Wake’s Presentation Because peptides overlap small- and large-molecule drugs in size, regulatory expectations differ by product size and clinical indication. Thus, analytical programs should be designed around critical quality attributes (CQAs)…
A Universal Assay Determination Method for Antisense Oligonucleotides: A New Slope Spectroscopy Method
Antisense oligonucleotides (ASOs) are short, synthetic, single-stranded oligodeoxynucleotides that can alter RNA and reduce, restore, or modify protein expression through several distinct mechanisms. ASO technology has become an important drug discovery platform for most major pharmaceutical companies. To date, six antisense drugs have been approved by regulatory agencies to treat diseases spanning viral infections, hyperlipidemias, and neurological diseases. More than 50 additional ASO drugs are in clinical trials. For an ASO drug product, an assay of its active pharmaceutical ingredient…
Nontargeted HCP Monitoring in Downstream Process Samples: Combining Micro Pillar Array Columns with Mass Spectrometry
Protein biopharmaceuticals have emerged as important treatments for diseases with otherwise unmet medical needs. These biologics are produced by recombinant mammalian, yeast, or bacterial expression systems. Along with therapeutic proteins, those cells produce endogenous host-cell proteins (HCPs) that can contaminate biopharmaceutical products despite multiple purification steps in downstream processing. Because such process-related impurities can affect product safety and efficacy, they need to be monitored closely. Multicomponent enzyme-lined immunosorbent assays (ELISAs) presently are the workhorse method for HCP testing, with high…
Making Safe and Effective CAR T Cells: How Droplet Digital PCR Can Improve Their Quality Control
Chimeric antigen receptor (CAR) T cells first entered US clinics in 2017 (1), and this therapeutic modality holds tremendous potential as one of the most effective forms of personalized cancer care ever to reach patients. The revolutionary impact of CAR T-cell therapy comes from its ability to rewire our own immune defenses to kill cancer cells: It essentially modifies a patient’s naturally existing immune cells to boost their recognition and attack of cancer cells so that the person’s own immune system…