The commercial manufacturing success of monoclonal antibodies (MAbs) has become a touchstone of the biopharmaceutical industry. MAbs are so well established that they often are referred to as “traditional” biologics, and well-known MAb processing methods have become a model for processing of other “advanced” or “emerging” therapies. But MAb processing continues to advance as biomanufacturers seek ways to improve efficiencies, lower costs, and (most recently) increase sustainability of facilities. Drug makers are particularly interested in strategies for MAb process intensification.…
Author Archives: Habib Horry
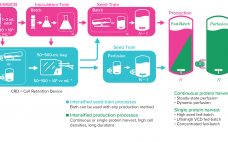
The Critical Role of Media in Intensified Upstream Processes
As the need for novel therapeutics increases, so does pressure on the biopharmaceutical industry to improve productivity, accelerate development, increase, and reduce costs — all while ensuring drug product quality. Upstream intensification strategies such as perfusion culture can address those challenges and achieve higher protein titers that can translate into higher throughput, improved flexibility, and compressed timelines. Successful implementation of perfusion culture or the transition to perfusion from fed-batch culture requires a different and strategic approach to media selection, not…