Design for manufacturing (DfM, also known as design for manufacturability) is a common approach in engineering industries when complex, multistep production processes are developed and installed to manufacture products. Adherence to DfM approaches has been prevalent for decades in the automotive, aerospace, and electronics industries, among others (1–3). Recently, a generalized manufacturability-assessment tool with strategies to weigh different aspects of manufacturing has been proposed with numerous similarities to that described herein specific to the field of bioprocess development (4). Although…
Author Archives: Konstantin Konstantinov
Long-Term Reliability of an Aseptic On-line Glucose Monitoring and Control System for Perfusion CHO Cell Culture
Among the variables that are appropriate for direct feedback control of the perfusion rate in mammalian cell cultures, high priority should be given to the glucose concentration. Here we describe the application of a closed-loop control scheme for the long-term cultivation of CHO cells in a high cell density (35 – 40 million cells/ml) perfusion process. The monitoring and control system worked successfully for 2.5 months without any signs of performance degradation. In targeting industrial applications, issues such as reliability,…
Development, Qualification, and Application of a Bioreactor Scale-Down Process: Modeling Large-Scale Microcarrier Perfusion Cell Culture
Qualified scale-down models of large-scale cell culture processes are essential to conducting studies for applications such as investigating manufacturing deviations, enhancing process understanding, and improving process robustness. For example, scale-down models can be used for raw material investigations as well as evaluation and qualification of new good manufacturing practice (GMP) cell banks for manufacturing implementation. Process characterization studies are performed also with qualified scale-down models to improve process consistency (1, 2). Often it is impractical to conduct investigational studies at…
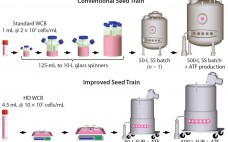
A Novel Seed-Train Process: Using High-Density Cell Banking, a Disposable Bioreactor, and Perfusion Technologies
A typical cell culture process begins with thawing of a cryopreserved cell-bank vial, followed by successive expansions into larger culture vessels such as shake flasks, spinners, Wave bags, and stirred bioreactors (1). When culture volume and cell density meet predetermined criteria, the culture is transferred to a production bioreactor in which cells continue to grow and express product. This approach presents several challenges. Shake flasks or spinners used in the initial stages require manual manipulations inside a laminar flow hood,…