Cell and gene therapies (CGTs) offer potential cures to some of the most challenging illnesses of our time. The number of such therapies approved for market is set to surge in the next 10 years (1). Yet current manufacturing approaches are not fit for purpose. Biomanufacturing must adapt to prevent the industry from unintentionally sleepwalking into causing harm to patients. Some urgently needed changes could come with learning about the mindset of the medical-devices industry. Background and Current State After…
Business
Microbiome-Based Therapeutics: Negotiating Key Development Challenges
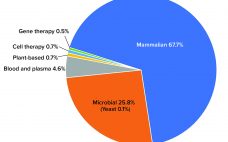
Microbiome-based therapeutics have evolved significantly in recent years, with several promising candidates advancing through the clinical pipeline. That progress is the result of growing evidence showing that targeting and manipulating the microbiome could improve human health and treat more than 25 conditions by restoring healthy bacterial populations (1). The human microbiome comprises a diverse community of microorganisms, helpful and harmful. It differs by person based on factors such as genetics, environmental influences, diet, and immune function. Microorganisms play a role…
eBook: ADCs — Evolving Links in the Biopharmaceutical Pipeline
Antibody–drug conjugate (ADC) developers both old and new are talking about the next generation of drug candidates coming through their pipelines. In April 2021, Zynlonta (loncastuximab tesirine, from ADC Therapeutics) became the eleventh such product to receive approval from the US Food and Drug Administration (FDA). But with dozens of ADC candidates currently in clinical trials, those 11 products represent the tip of the ADC iceberg. In this eBook, Dan Stanton (founding editor of BioProcess Insider) explores ADC production history,…
Total Global Capacity Finally Shows Improved Productivity
Since 2018, global bioprocessing capacity has grown from 16.5 million liters (1) to 17.4 million liters. Although output has continued to expand at around 12% overall, that rate represents a significant slowing in capacity growth as the industry moves toward greater productivity and efficiency. Trends that we have tracked in the BioPlan Associates annual report of biopharmaceutical manufacturing capacity and production (2) for over 17 years correlate with that finding. Titers are increasing; single-use technologies have reduced the need for…
Untapped Potential of Tissue Engineering: The Three Obstacles Holding It Back
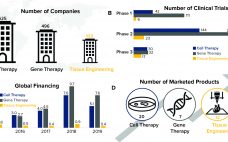
Regenerative medicine is the interdisciplinary field comprising tissue engineering, cell therapy, and gene therapy. These biopharmaceutical modalities, also referred to as advanced therapies, are growing rapidly, characterized by groundbreaking therapeutic advances that have the potential to change how healthcare providers deliver care. As Figure 1 shows, cell and gene therapies have gained traction over the past decade, as evidenced by large increases in investment and the number of marketed products. By contrast, tissue engineering investment and product commercialization has lagged…
Manufacture and Regulation of Cell, Gene, and Tissue Therapies: Part 2 — Regulatory Guidances
The US Food and Drug Administration (FDA), the European Medicines Agency (EMA), the Medicines and Healthcare Products Regulatory Agency (MHRA), and Japan’s Pharmaceutical and Medical Devices Agency (PMDA) all offer support and guidance for developers of advanced therapy medicinal products (ATMPs). Some agencies have issued guidelines to help companies through different stages of product development — from research and development to marketing authorization and postauthorization activities. Such guidelines are updated regularly as more knowledge becomes available from the development and…
Successful biosimilar development requires an outsourcing partner with the right experience
Demand for biosimilars is expected to increase significantly in the coming years, particularly in the United States. Developing new biosimilar candidates is a challenging endeavor that requires partnerships with outsourcing partners that have deep protein science, analytics, and process engineering expertise. Avid Bioservices has successfully supported biosimilar development for nearly a decade and is further expanding its capacity to provide manufacturing services from the bench through larger-scale commercialization. Anticipating Strong Growth in Demand for Biosimilars The value of the global…
Trade-Secret Vulnerabilities: Recent Hacking Schemes Highlight the Need to Protect Proprietary Pharmaceutical Information
On 21 July 2020, the US Attorney’s Office in Spokane, WA, unveiled a sweeping indictment accusing a pair of Chinese hackers of conspiring to steal trade secrets from a number of American pharmaceutical companies and research institutions, including biotechnology companies working on potential COVID-19 vaccines (1). Although the hackers apparently did not succeed in their attempt to obtain vaccine data, their years-long conspiracy involved other attacks on the pharmaceutical industry — and netted them troves of sensitive commercial information. Among…
Biomanufacturing Workforce Development: Fostering Talent During and After the Pandemic
Beyond the human suffering and economic damage caused by COVID-19, one of the most powerful results of the pandemic has been to focus global attention on drug and vaccine development for infectious diseases. Massive investments by governments, institutions, and biopharmaceutical companies have accelerated development of novel therapeutics, including messenger RNA (mRNA) and viral-vector vaccines, that are poised to become transformational platform technologies for biopharmaceutical manufacturing (1). Furthermore, the well-established technology of monoclonal antibodies (MAbs) has lived up to its promise…
Emerging Strategies for Drug-Product Comparability and Process Validation: Part 2 — Validation, Legacy Products, and Lifecycle Management
This two-day CASSS CMC Strategy Forum explored many technical, practical, and regulatory facets of biological drug-product (DP) analytics, process validation, and comparability. Part 1 of this report summarized the discussions on drug-product analytics and comparability in BPI’s March 2021 issue (1). Here we report on day two presentations and discussions on validation, legacy products, and lifecycle management. Session Three: Drug-Product Validation The morning session focused on principles of process validation with examples of challenges specific to drug products. New Risk-Based…